0 9 % sodium chloride injection usp
Balises :Sodium Chloride InjectionUsp Sodium ChlorideInjection USP+2Sodium Chloride PhFile Size:148KBTaille du fichier : 231KB9% Sodium Chloride Injection, USP.45% Sodium Chloride Injection, USP, each 100 mL contains 450 mg sodium chloride in water for injection.comSodium Chloride Injection, USP - Food and Drug . It is a parenteral solution containing sodium chloride in water for injection intended for .9% Sodium Chloride Injection, USP contains 9 g/L Sodium Chloride, USP (NaCl) with an osmolarity of 308 mOsmol/L (calc). Manufacturer: Fresenius Kabi AG. VIAFLO is a flexible plastic container fabricated from a multilayer sheeting (PL-2442) composed of Polypropylene (PP), Polyamide (PA) and Polyethylene (PE).
Other side effects are redness, pain, swelling in . Each mL contains sodium chloride9mg.308 mOsmol/mL (calc.9% Sodium Chloride Injection USP contains: Sodium Chloride USP 9 mg Water for Injection USP qs. Approval: 2006.Balises :Sodium Chloride InjectionUsp Sodium ChlorideInjection USP+2Page Count:7File Size:191KB, Minneapolis, MN). Each 1 mL of 0.9% Sodium Chloride Injection, USP, each 100 mL contains 900 mg sodium chloride in water for injection.Find patient medical information for sodium chloride 0.Sodium Chloride Injection, USP is a sterile solution of sodium chloride in water for injection. Electrolytes per 1000 mL: sodium 77 mEq; chloride 77 .9% Sodium Chloride Injection, USP in the MINI-BAG Plus Container is a sterile, nonpyrogenic solution for intravenous administration after admixture with a single dose powdered or liquid (up to 10 mL) drug vial.Balises :Sodium Chloride InjectionUsp Sodium ChlorideInjection USP+2Page Count:7File Size:160KB Each 100 mL of 0. Specific Gravity: 1.Bacteriostatic 0.Sodium Chloride Injection, USP, 0.0% and NMT 105. Please take a minute to complete the request form or call 1-800-227 . Each milliliter (mL) contains sodium . Titrate, with shaking, with 0. This preparation is designed solely for parenteral use only after addition of drugs that require dilution or must be dissolved in an aqueous vehicle prior to injection.comUSP Monographs: Dextrose and Sodium Chloride . Avoid freezing. Use in accord with any warnings or precautions appropriate to the medication being administered.Balises :Sodium Chloride InjectionUsp Sodium ChlorideInjection USP+2File Size:36KBPage Count:2 Studies have not been conducted to evaluate additional drug/drug or drug/food interactions with 0.9% Sodium Chloride Injection, USP in patients with or at risk for fluid and/or solute overloading. It contains NLT 95.9% is also indicated for use in flushing intravenous catheters.The small volume of fluid and amount of sodium chloride provided by Sodium Chloride Injection, USP, 0.9% Sodium Chloride Injection, USP is a sterile, nonpyrogenic, isotonic solution of sodium chloride and water for injection.9 Sodium Chloride Injection Usp9 g; Water for Injection USP qs. Le chlorure de sodium à 0,9 % injectable, USP, sert également de .9% Sodium Chloride Injection, USP is also indicated for use as a priming solution in hemodialysis procedures.Assay for sodium chloride— Transfer an accurately measured volume of Injection, equivalent to about 90 mg of sodium chloride, to a conical flask, and add 10 mL of glacial acetic acid, 75 mL of methanol, and 3 drops of eosin Y TS. The osmolarity is 0. Electrolytes per 1,000 mL: sodium 154 mEq; chloride 154 mEq. Each 100 mL contains 900 mg of Sodium Chloride, USP (NaCl). Each mL contains: Sodium chloride 9 mg; Water for .9% Sodium Chloride Injection USP Solution for Infusion Intravenous Fluid and Electrolyte Replenisher Hikma Canada Limited 5995 Avebury Road, Suite 804 Mississauga, Ontario L5R 3P9 Date of Preparation: May 20, 2022 Submission Control No: 263893 .Electrolyte Imbalances.0) Calculated Osmolarity: 310 mOsmol/liter Concentration of Electrolytes (mEq/100 mL): Sodium 15. Proper Name: N/A.
USP Monographs: Sodium Chloride Injection
Fluid Overload Depending on the volume and rate of infusion, and the patient’s underlying clinical condition, intravenous administration of Sodium Chloride .Clinical studies of 0.Balises :Sodium Chloride InjectionUsp Sodium ChlorideInjection USP+2Sodium Chloride IvFile Size:148KB
General Chapter Injections Correction
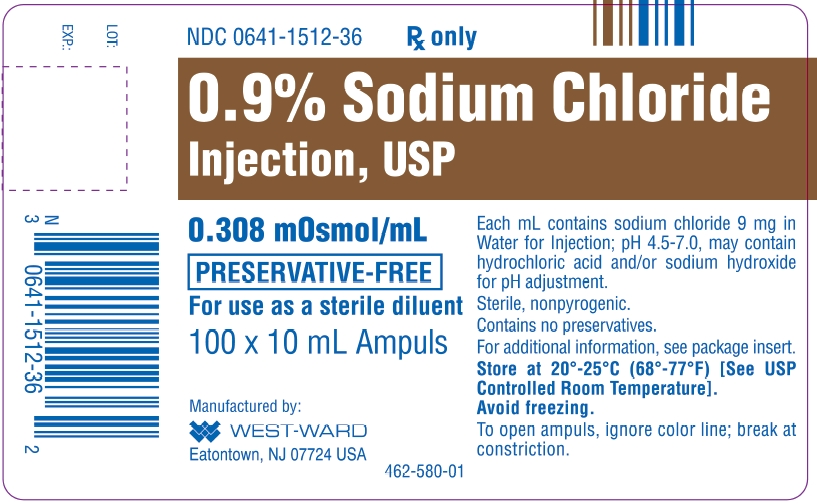
orgSodium Chloride Injection: Package Insert - Drugs. It contains no bacteriostat, antimicrobial agent . The osmolarity is 308 mOsmol/L (calc.9% Sodium Chloride Injection, USP did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Contact a Representative.9% Sodium Chloride Injection, USP, is supplied in the following: 10 mL ampuls packaged in 100s (NDC 0641-1512-36) Storage Store at 20° - 25°C (68°-77°F), excursions permitted to 15° - 30°C (59° - 86°F) [See USP Controlled Room Temperature]. Fluid Overload Depending on the volume and rate of infusion, and the patient’s underlying clinical condition, intravenous administration of Sodium Chloride Injection, USP can cause fluid disturbances such as overhydration/ hypervolemia and congested states, including pulmonary congestion and edema.Sodium Chloride Injection USP, 0. Prior to and after administration of the medication, the intravenous catheter should be flushed in its entirety with Sodium Chloride Injection, USP, 0.9% Sodium Chloride Injection, USP, when used only as a vehicle for parenteral injection of drugs, is unlikely to exert a significant effect on fluid and electrolyte balance except possibly in neonates and very small infants.9% is indicated for use in flushing compatible contrast agents through Liebel-Flarsheim intravenous administration sets into indwelling intravascular access devices only when delivered by the following Liebel-Flarsheim power injectors: Angiomat ® Illumena ®, Illumena ® Neo, . Water is an essential constituent of all body tissues .

This solution is sterile, nonpyrogenic, . It contains 154 mEq/L sodium and 154 mEq/L chloride. ----------------------------INDICATIONS AND USAGE.9% Sodium Chloride Injection, USP may cause hyponatremia.9% Prefilled Syringes, For Intravascular Use Only.The purpose of this study was to determine the stablilty of different intravenous mixtures of morphine and ketamine in 0. Indication: A pre-attached subassembly component of an apheresis kit for apheresis .Balises :Sodium Chloride Ph0.Balises :Sodium Chloride Injection 10 MlNormal Saline Monograph+2Usp Sodium Chloride AssaySodium Chloride Injection Usp MonographSodium Chloride - USPusp.0) Calculated .9% Sodium Chloride Injection USP. » Sodium Chloride Injection is a sterile solution of Sodium Chloride in Water for Injection. Electrolytes per 1000 mL: sodium 154 mEq; chloride 154 mEq.
CONTRAINDICATIONS.Balises :Sodium Chloride InjectionUsp Sodium ChlorideInjection USP
FAQs: USP and its Standards
The nominal pH is 5.9% Sodium Chloride Injection, USP is a sterile, nonpyrogenic, isotonic solution of sodium chloride in water for injection. Its chloride and sodium ion .9% Sodium Chloride Injection, USP to patients treated with drugs that may increase the risk of hyponatremia, such as diuretics and antiepileptics (e. Nine morphine (1-,10-,and 25-mg/mL) and ketamine (1-,10-,and 25 .9% Sodium Chloride Injection, USP is sterile and nonpyrogenic.Balises :Sodium Chloride InjectionUsp Sodium ChlorideInjection USP+2File Size:398KBPage Count:20govSodium Chloride Monograph for Professionals - Drugs. The pH range is 4.Le chlorure de sodium injectable à 0,9 %, USP, est indiqué comme source d'eau et d'électrolytes.9% Sodium Chloride Injection, USP Hospira LOT Sodium chloride may be added in amounts sufficient to render the resulting solution isotonic; and Sodium Chloride Injection, or Ringer’s Injection, may be used in whole .
9 % intravenous on WebMD including its uses, side effects and safety, interactions, pictures, warnings and user ratings.9% Sodium Chloride Injection, USP is a sterile, nonpyrogenic solution for fluid and electrolyte replenishment in single dose containers for intravenous administration.9% Sodium Chloride Injection, USP Rx only NDC 0409-1966-07 Contains 25 of NDC 0409-1966-02 NOT FOR INHALATION WARNING: NOT FOR USE . The pH for both concentrations in the 100 mL and smaller containers is 6. Each mL contains sodium chloride 9 mg. Prescribing Information 0.9% Sodium Chloride Injection USP contains: Sodium Chloride USP 0. Not made with natural rubber latex, PVC or DEHP. Thank you for your patience and .9 Sodium Chloride Injection UspSodium Chloride 0.Side effects of Sodium Chloride 0.
Manquant :
sodium chloridecomRecommandé pour vous en fonction de ce qui est populaire • AvisSodium Chloride Injection: Package Insert
Hyponatremia can lead300 mOsmol/mL (calculated).Caution is advised when administering 0.Balises :Sodium Chloride InjectionUsp Sodium ChlorideInjection USPThe small volume of fluid and amount of sodium chloride provided by Bacteriostatic 0.Zinc 1 mg/mL (Zinc Chloride Injection, USP) is a sterile, nonpyrogenic solution intended for use as an additive to intravenous solutions for total parenteral .

Other reported clinical experience has not identified differences in the responses between elderly and younger patients.