1n vs 1m naoh
Standardization of 0. 35256-6X1LEA 159 EUR. Ratings: Total Ratings: 0 Avg. † For reduction to below detection limit of 10 .Regarder la vidéo2:39in this animation you will learn 1 normal solution of sodium hydroxide.5 1 h 22 * For reduction to below detection limit of < 3 organisms/mL. University of Sharjah. « Directives de prise en charge médicale de l'hydroxyde de sodium (NaOH) ». Par produit actif, tu dois entendre H+, OH-, 1mole . Preparation of 10 M Sodium Hydroxide from 50% (w/w) Stock Solution. In normality, another factor, stoichiometry, is added to molarity. Ratings: Ecrire un avis.Temps de Lecture Estimé: 3 min ⬇ Scroll down to see reaction info and a step-by-step answer, or balance another .95 g of the dried KHC8H4O4, and transfer to a 250-ml conical flask. Molar mass of NaOH = 39.NaOH: Quantity: 1 L: Formula Weight: 4: Packaging: 1M Solution: Chemical Name or Material: Sodium Hydroxide Solutions, 1M/1N: Documents Safety and Handling ShelfLife: 2 years: DOTInformation: DOT Class 8, II, : Corrosive: SDS.Auteur : Spectrum Classes Pour préparer ces recettes, commencez avec 1 litre d'eau et incorporez lentement le NaOH solide. Catalog number: 124260010., Bp, Fisher Chemical™ Fornecedor: Honeywell Chemicals. A 1 N solution is one in which exactly 1 equivalent of solute is dissolved in a total solution volume of exactly 1 L.Recettes pour les solutions de NaOH courantes. Total Evaluation: 0 Moyenne des Notes : 0. Normality is defined as molarity multiplied by a stoichiometric factor z. Your price: Sign In.5g/L H 2 SO 4-> 2·H + + SO 4 2-98 g/mol 2N = 1M = 98g/L NaOH -> Na + + 1·OH-40 g/mol 1N = 1M = 40g/L Synonyme(s): Hydroxyde de .For NaOH, it is 1, so 1N for NaOH means the same as 1M, i.What Is Molarity?
unité 1N
Example for factor calculation for standardization of 1 m . Sodium hydroxide.
NF Monographs: Sodium Hydroxide
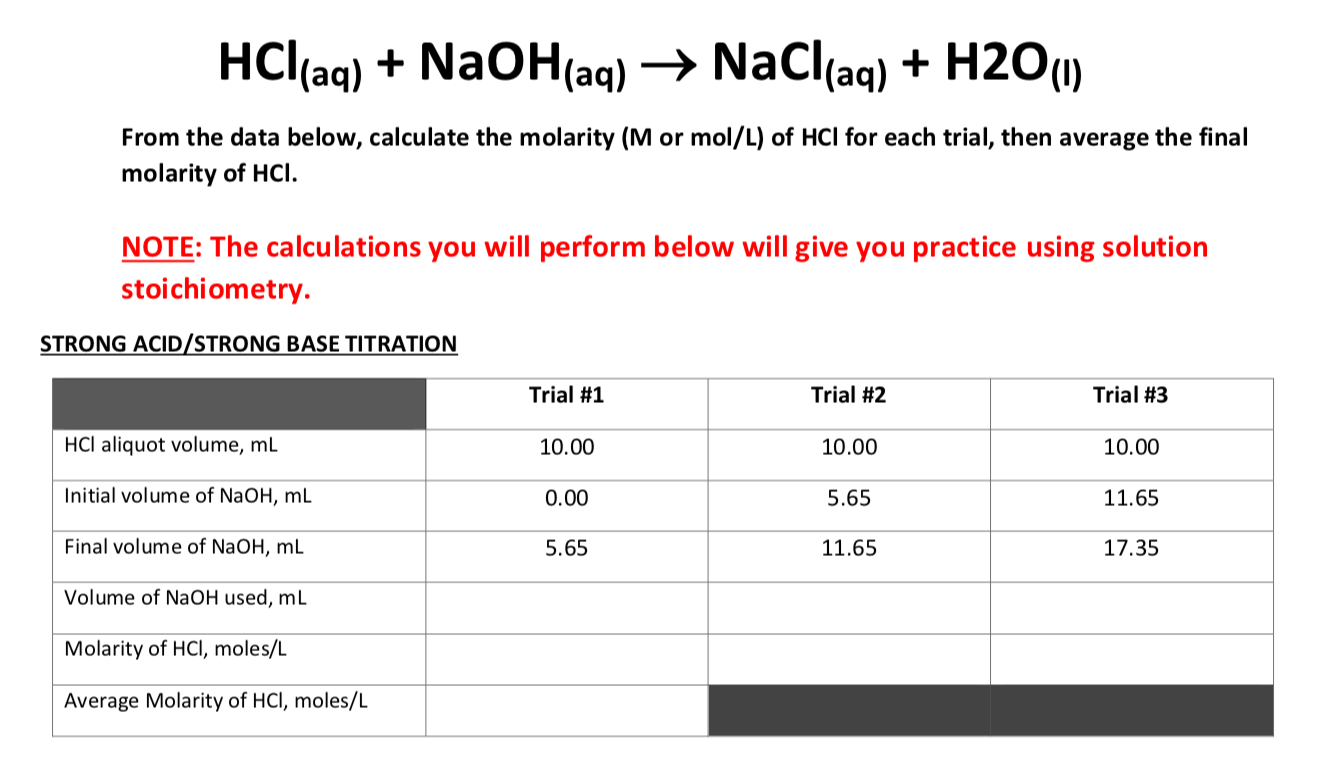
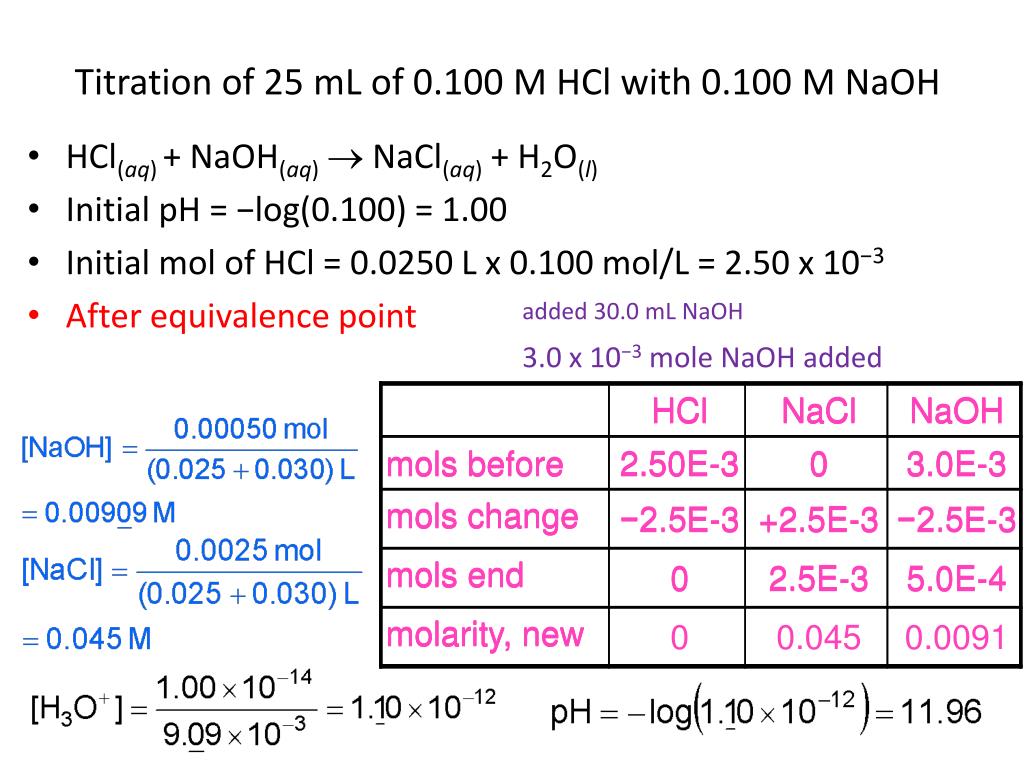
This is the volume that results after V 1 from the stock solution has been diluted with diluent to achieve a total diluted . HCl + NaOH → NaCl + H 2 O.
Balanced Chemical Equation.1 mol/L il est nécessaire dissoudre 4 g NaOH (La puretré est de 100 %) de la .Molarity is defined as the moles of a solute per liter of a solution 1M NaOH solution means 1 Mole of NaOH dissolves in 1 liter CO, free water (solvent) 1Mole of NaOH = Molecular weight of NaOH. volumetric solution, Fluka™.
Acid & Base Normality and Molarity Calculator
Sodium hydroxyde 1M NaOH (1N) en solution aqueuse Reag. Therefore, the solution in question would contain 3. Step 1: Using the digital balance weigh accurately 40g of sodium hydroxide (NaOH). Once you realize there are two sodium ions per carbonate ion, the problem is simple: N = 0. Molarity is given by M = n V where, M is the molarity n is the number of moles of solute V is the volume of solution (in L) 1M solution means 1 mole of a substance per liter of solution. Molarity is defined as number of moles per liter of solution. Price (USD) Price: 37. Ankur Choudhary is India's first professional pharmaceutical blogger, author and founder .Mol/L et N - Futuraforums. Convert between NaOH .This means the 10N solution of sodium hydroxide is also a 10M solution.Cách pha NaOH 1N.Prenez environ 1M de NaOH, 25 ml dans une fiole conique, ajoutez 2 à 3 gouttes de indicateur de phénolphtaléine et titrer avec une solution standard dacide oxalique .Auteur : source of skills
HCl + NaOH = NaCl + H2O
Sodium hydroxide 1 M NaOH (1N) in aqueous solution Reag.20423 x ml NaOH solution Also see: Determination of Shelf Life of Solutions in Laboratory. Sodium hydroxide 1 . Ce M1 est la molarité exacte de la .Sodium hydroxyde 0. BUT, have only 250 ml of the solution, which is 1/4 of a liter. M1 × 25 = 1 × V ml dacide requis pour neutri . 35256-6X1L 35256 . Fórmula: NaOH.Meilleure réponse.1N means that the NaOH solution in 0. Dear Dr Waleed, Dissolving 40. Synonyms: Caustic soda.321 g Na 2 CO 3 x (1 mol/105.
What Is the Difference Between Molarity and Normality?
A mole of HCl is 36.Comment calcule-t-on le pH de 1 mol/l NaOH, 1M NaOH et 1N . Headline Comment: Standardised against Hydrochloric Acid 1M.Sodium hydroxide concentrate 0.
Is 1N NaOH same as 1M NaOH?
com/playlist?list=PLLdtmjp5gXcuVENGoPfd8pdpKmWHExYL_#NaOH How to #prepare 0. solution volumétrique, Fluka™ Imprimer.Normality, N, expresses a concentration of a solution.This calculator provides lab-ready directions describing how to prepare an acid or base solution of specified molarity (M) or normality (N) from a concentrated acid or base .Normality is defined as the number of equivalents of solute dissolved per liter of solution (equivalents/L = N) (Equations 1, 3, and 4).1 M NaOH in water (0.Volumetric standard solution, titrated at 25°C to pH 8. Quality Name: volumetric solution. The preparation process of a 10M solution has been described in the following posts: Preparation of 10 M Sodium Hydroxide (NaOH) Solution from NaOH pellet.1 N Sodium Hydroxide.646 gm per liter.0 g of NaOH in one liter volumetric flask gives abiut one molar solution. Chúng ta sẽ áp dụng công thức Cm=n/V, trong đó n=m/M.Sodium hydroxide, pure, 1N Standard Solution, Thermo Scientific Chemicals. Synonyms: Hydroxyde de sodium. Crush 1 to 2 g of primary standard potassium hydrogen phthalate (KHC8H4O4) and dry in a glass container at 120°C for 2 h. To solve this problem, you need to know the formula for sodium carbonate. How one can prepare dilute solution from stock solution using normality equation., it is the equivalent concentration.01 2 h 4 or 22 S. Densidade: 1,04 g/cm³ (20 °C)For acid reactions, a 1 M H 2 SO 4 solution will have normality (N) of 2 N because 2 moles of H+ ions are present per liter of solution.1 moles HCl per liter.Molar mass of NaOH (Sodium hydroxide) is 39. = Vml ÷ 25 = XM .Re : unité 1N.
How to Calculate Normality of a Solution
Quantity-Request bulk or custom . Classer par: Attention. solution volumétrique, Fluka™ Marques : Honeywell Chemicals.
Cách pha NaOH 1N
Peso Molecular: 40 g/mol. Weight accurately 0.1N NaOH or NaOH 0.

In order to solve a normality calculation for NaOH, the equivalent weight .1N), Eluent concentrate for IC; CAS Number: 1310-73-2; Synonyms: Sodium hydroxide solution; Linear Formula: NaOH; find Supelco-43617 MSDS, related peer-reviewed papers, technical documents, similar products & more at Sigma-Aldrich5 1 h 4 or 22 A.
Find the normality of 0.Regarder la vidéo8:49This video contains detail procedure for preparation of 1M NaOH solution as well as the basic principle involved in standardization of prepared NaOH solution.5 g/mol 1N = 1M = 36. Vậy chúng ta có Cm=m/ (M x V) => m= Cm x M x V.The normal concentration of a solution (normality, CN) is always equal to or greater than the molar concentration (molarity, CM) of the solution. This compound is also known as Sodium Hydroxide.
What is the Difference Between Molarity & Normality?
Giả sử nồng độ ở đây là 1N, thể tích là 1L; M của NaOH là 40. Ponto de Fusão: 0 °C.Most recent answer. solução volumétrica, Fluka™. 1M NaOH vs acide oxalique . subtilis spores 1. Bonsoir Merins, Un solution normale est une solution qui contient 1mole par litre de produit actif.Regarder la vidéo6:49In this video I have shown how to prepare 1N NaOH solution in 100 ml. 35256-6X1L 35256-1L 35256-10L-VP 35256-5L-VP 35256-4X5L 35256-5L.Nous voudrions effectuer une description ici mais le site que vous consultez ne nous en laisse pas la possibilité.Procedure for Preparing 1N NaOH Solution.0 percent of Na 2 CO 3. How would you prepare 500mL of a 1N solution of NaOH?
Dilution Calculator
Therefore, 1M HCl is the same as 1N HCl, but when we take . Do đó, ta cần lấy m= 1 x 40 x1 = 40g của NaOH.N vs M HCl -> 1·H + + Cl-36. Product Name: Sodium Hydroxide 1 mol/l (1N) volumetric solution. Sort all by: Danger. Normality is defined as the gram equivalent weight per liter of solution. Sodium hydroxide [ 1310-73-2 ]. What is the pH of 1N NaOH solution? pH of 1 N NaOH . Open in App.

[Updated!]
Specifications: Titer at 20°C: 0. in gm of potassium biphthalate M= ----- 0. 1 L, Plastic Bottle, Each. Sodium Hydroxide.V 2 is the final volume of the diluted solution. Un agitateur magnétique est utile si vous en avez un. Equation is already balanced. Since the molar mass of NaOH is 40 g/mol, the concentration is 40 g/L. Ibrahim Abdel-Rahman.NaOH molecular weight.1N) ≥99% solution volumétrique, Fluka™ Imprimer.
Preparation and Standardization of 1M NaOH
Check Playlist Pharma Analysis Subject For B Pharma 1st Sem - https://youtube.0 percent and not more than 100.Achetez Solution d’hydroxyde de sodium 1 M (1N), solution étalon NIST prête à l’emploi, pour l’analyse volumétrique, conforme aux exigences analytiques de Ph. Therefore, 1M NaOH means 1 mole of NaOH per liter .NF Monographs: Sodium Hydroxide.Auteur : Amit Lunkad Titrate with NaOH solutions. Stopper the containern andcool in a desiccator. Hướng dẫn cách pha dung dịch NaOH 1N từ NaOH bột. Likewise, for a 0.Solution d’hydroxyde de sodium 1 M (1N), solution étalon NIST prête à l’emploi, pour l’analyse volumétrique, conforme aux exigences analytiques de Ph.5 1 h 4 or 22 B.Organism NaOH (M) Time* Temp. For sulfide precipitation .

moles NaOH to grams. Formula: NaOH MW: 40 g/mol Boiling Pt: 100 °C Melting Pt: .1N) ≥99% solution volumétrique, Fluka™ Marques : Honeywell Chemicals. volumetric solution, Fluka™ Supplier: Honeywell Chemicals.Hidróxido de sódio 1 M NaOH (1N) em solução aquosa Reag.Quel est le pH de NaOH 1 mol/L, NaOH 1N et NaOH 1M?Le pH de 1mol/L NaOH, 1N NaOH et 1M NaOH est de 14.1 1 h 4 or 22 C. Caution—Exercise great care in handling .321 g sodium carbonate in a 250 mL solution. Ponto de ebulição: 100 °C.futura-sciences.
The molecular weight of NaOH = The sum of atomic masses of all atoms in a molecule. M is denoted for molarity. Get control of 2022! Track your food intake, exercise, sleep and meditation for free. solution volumétrique, Fluka™ Fournisseur: Honeywell Chemicals.5 percent of total alkali, calculated as NaOH, including not more than 3.What do you mean by 1N NaOH solution? To make a 1N solution of NaOH, 40 grams of NaOH are dissolved in 1 L.Pour la préparation de 1000 mL de la solution Hydroxyde de sodium de concentration de 0. Sodium hydroxyde 0. Step 2: Add precisely weighed NaOH .0 48 h† 22 B.