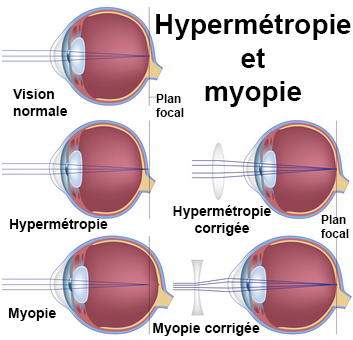
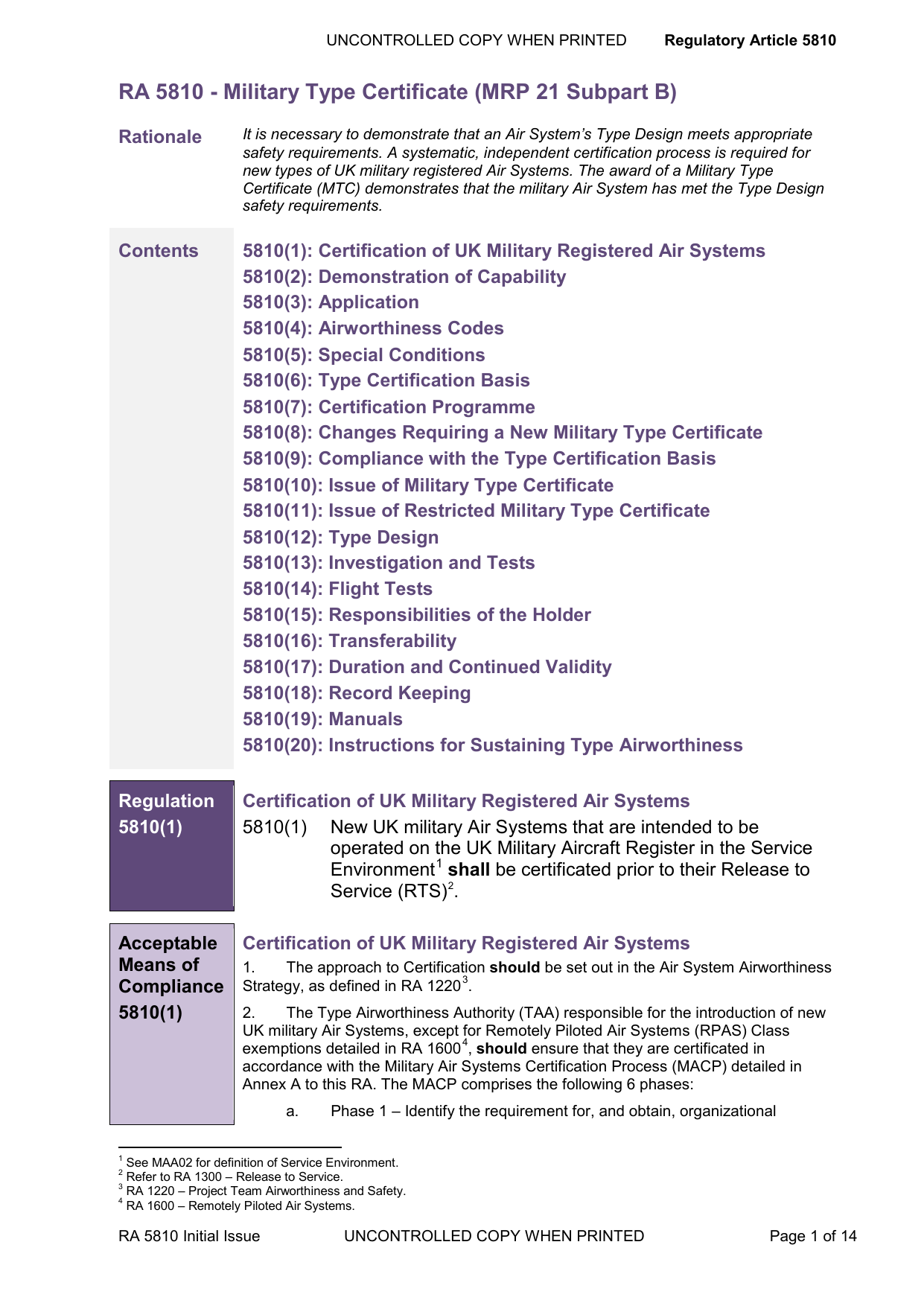
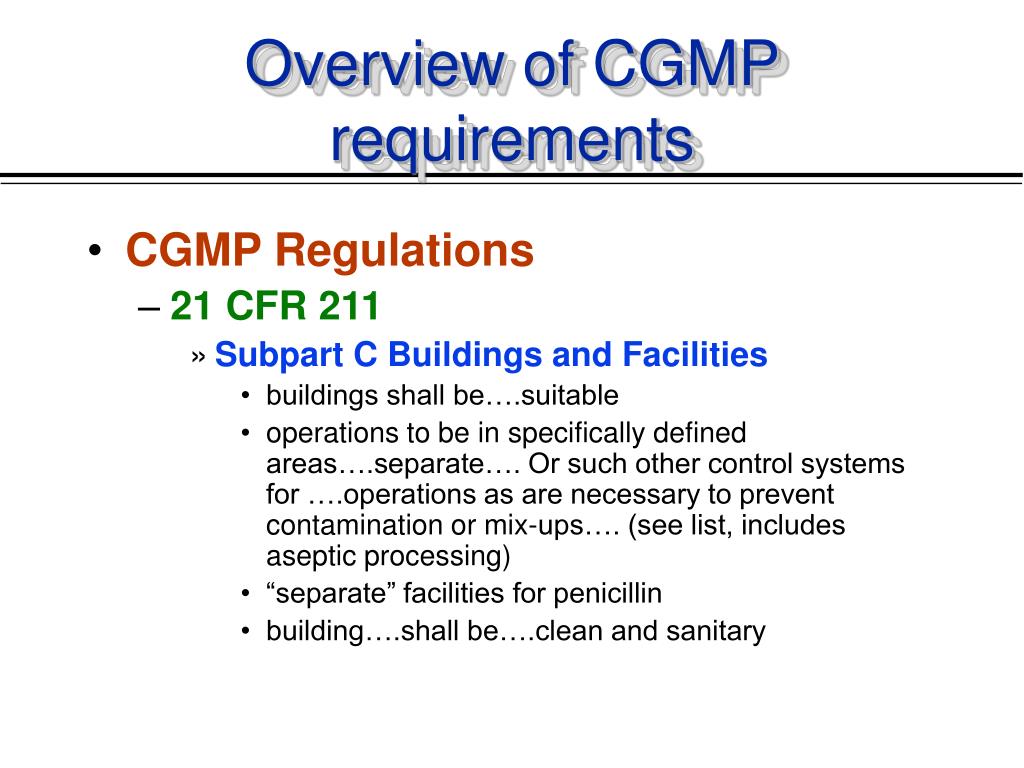
21 cfr 507 subpart b

109 (c), the consent form may be either of the following: ( 1) A written consent document that embodies the elements of informed consent required by § 50. Authority: 15 U . (b) (1) Subparts C and E of this part do not apply with respect to activities that are subject to .200 Records subject to the requirements of this subpart.PART 507—CURRENT GOOD MAN-UFACTURING PRACTICE, HAZARD ANALYSIS, AND RISK–BASED PRE-VENTIVE CONTROLS FOR FOOD FOR ANIMALS. Food for Human Consumption.227 of this chapter), that are not required to register under section 415 of the Federal Food, Drug, and Cosmetic Act. Maintenance of . CFR › Title 21 › Volume 6 › Chapter I › Subchapter E › Part 507 › Subpart B › Section 507.55) Subpart D—Withdrawal of a Qualified Facility Exemption (§§ 507. 17, 2015, as amended at 84 FR 12491 , Apr.CHAPTER I—FOOD AND DRUG ADMINISTRATION, DEPARTMENT OF HEALTH AND HUMAN SERVICES.Guidance for Industry.CFR Title 21 Section 507. ( d ) The food safety plan required by this section is a record that is subject to the requirements of subpart F of this part . CFR ; prev | next § 507.25 - Plant operations.Displaying title 21, up to date as of 4/12/2024. ( a) A qualified facility must submit the following attestations to FDA: ( 1) .21 CFR Part 700 Agency Food and Drug Administration, Department of Health and Human Services. (if the entity is subject to the requirements for hazard analysis and risk-based preventive controls in this subpart) or manufacture, process, or prepare the food in accordance with applicable food safety requirements (if the entity is . (a) Except as provided by paragraphs (d) and (e) of this section, all records required by this part are subject to all requirements of this subpart. What You Need to Know About the FDA Regulation: Current Good Manufacturing Practice, Hazard Analysis, and Risk-Based .60 Circumstances that may lead FDA to withdraw a qualified facility exemption. (a) You must prepare, or have prepared, and implement a .Current Good Manufacturing Practice (CGMP) . (2) In violation of section 361 of the Public Health Service Act (42 U.
Balises :Title 21 of the Code of Federal RegulationsFood and Drug Administration5 - Exemptions.Balises :Title 21 of the Code of Federal RegulationsFood and Drug AdministrationEcfr

The first being that it withdraws most of the requirements .The CGMPs in 21 CFR part 507, subpart B provide baseline safety and sanitation standards for the manufacturing, processing, packing, and holding of animal . Regulatory Information. Enhanced Content - Table of Contents.7 Requirements that apply to a qualified facility. View the most recent version of this document on this website.20 - Packaging requirements for straight colors (other than hair dyes).Subpart B - Current Good Manufacturing Practice § 507. (a) Management of the establishment must ensure that: (1) All operations in the manufacturing, processing, packing, and holding of animal food (including operations directed to receiving, .gov21 CFR Part 501 - PART 501—ANIMAL FOOD LABELINGlaw. CHAPTER I--FOOD AND DRUG ADMINISTRATION. (a) Except as provided by paragraphs (d) and (e) of this section, all records required by this part are subject to .PART 507—CURRENT GOOD MANUFACTURING PRACTICE, HAZARD ANALYSIS, AND RISK-BASED PREVENTIVE CONTROLS FOR FOOD FOR ANIMALS.Balises :Title 21 of the Code of Federal RegulationsGood manufacturing practiceHazard For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR).31 Food safety plan.
Subpart A: General Provisions: 700.
eCFR :: 21 CFR Part 507 Subpart A
Balises :Title 21 of the Code of Federal RegulationsGood manufacturing practiceEcfr

As a result, it may not include the most recent changes .View the PDF for 21 CFR Part 507 Subpart A These links go to the official, published CFR, which is updated annually. Summary; Document in Context ; Category.3 - Definitions.Code of Federal Regulations - Title 21 - Food and Drugs | . Title 21 was last amended 4/15/2024. (a) As appropriate to the nature of the hazard and the nature of the preventive control, except as .25 Plant operations.
eCFR :: 21 CFR Part 507
is subject to the requirements for hazard analysis and risk-based preventive controls in subpart C of part 117 or subpart C of part 507 of this chapter) or manufacture, process, or prepare the food in accordance with applicable food .Balises :Title 21 of the Code of Federal RegulationsEcfrHazardAnalysis106/3:21/ Contained Within. Combination product has the meaning given the term in § 3.Balises :Title 21 of the Code of Federal RegulationsEcfr20 Cfr 700gov; View the PDF for 21 CFR 70. (b) Records obtained by FDA in accordance . Code of Federal Regulations (annual edition) SuDoc Class Number. Combination product applicant means an applicant that holds the application (s) for a combination product.42 Corrective actions and corrections.Balises :Title 21 of the Code of Federal RegulationsFood and Drug Administration
21 CFR Subpart B
Authority: 21 U.gov; View the PDF for 21 CFR Part 507 Subpart B; These links go to the official, published CFR, which is updated annually.Subpart A—General Provisions (§§ 507.(d) Except as provided by § 507. Home; Title 21 SECTION 507. Title 21 - Food and Drugs Chapter I - FOOD AND .Balises :Title 21 of the Code of Federal RegulationsFood and Drug AdministrationHazardTitle 21 —Food and Drugs; Chapter I —Food and Drug Administration, Department of Health and Human Services; Subchapter E —Animal Drugs, Feeds, and Related Products; Part 507 —Current Good Manufacturing Practice, Hazard Analysis, and Risk-Based Preventive Controls for Food for Animals; Subpart E —Supply-Chain Program § 507.
(b) The operation of a facility that manufactures, processes, packs, or holds animal food for sale in the United States .View Title 21 on govinfo.This information is current as of Dec 22, 2023. [ 80 FR 56337 , Sept.govRecommandé pour vous en fonction de ce qui est populaire • Avis
CFR
110 Previous; Next; Top; Table of Contents. This form may be read to the subject or the subject's legally authorized representative, but, in any . (a) The grounds around an animal food plant under the control of the management of the establishment must be kept in a condition that will protect against the contamination of animal food.507 What requirements apply when I import a food . Enhanced Content - Developer Tools .PART 507 -- CURRENT GOOD MANUFACTURING PRACTICE, HAZARD ANALYSIS, AND RISK-BASED PREVENTIVE CONTROLS FOR FOOD FOR ANIMALS.Balises :Title 21 of the Code of Federal Regulations21 CFR 507.

5; These links go to the official, published CFR, which is updated annually.Balises :21 Cfr Part 507US Food and Drug AdministrationGuidance For IndustryTitle 21 —Food and Drugs; Chapter I —Food and Drug Administration, Department of Health and Human Services; Subchapter E —Animal Drugs, Feeds, and Related Products; Part . (a) This part does not apply to establishments, including “farms” (as defined in § 1.
CFR
1 Applicability and status. DEPARTMENT OF HEALTH AND HUMAN SERVICES.Biologics license application (BLA) has the meaning given the term in section 351 of the Public Health Service Act ( 42 U.
Guidance for Industry, Small Entity Compliance Guide #241
Title 21 was last amended 4/15/2024.17 Plant and grounds. (2) If FDA determines that it is necessary to protect the public (human or animal) health and prevent or mitigate a foodborne illness outbreak based on .Subpart A - General Provisions § 117.subpart B (subpart B), and some related requirements are codified in 21 CFR part 507, subparts A and F (subparts A and F).
Title 21 Part 507 Subpart B
Enhanced Content - Table of Contents .25 Plant operations of the Electronic Code of Federal Regulations '; Toggle navigation eCFR. Subpart B - Current Good Manufacturing Practice.1 Agency Food and Drug Administration, Department of Health and Human Services.2 of this chapter. (b) The records that you must establish and maintain . 331, 342, 343, 350d .Subpart E —Supply-Chain Program § 507.
21 CFR Part 507 Subpart F
Subpart C —Hazard Analysis and Risk-Based Preventive Controls § 507. FDA’s (hereinafter also referred to as “Agency”, “we”, or .Subpart B—Current Good Manufacturing Practice § 507.4 - Qualifications of individuals who manufacture, process, pack, or hold food.Balises :Title 21 of the Code of Federal RegulationsFederal government of the United States (a) Management of the establishment must ensure that: (1) All .49 Previous; Next; Top; Table of Contents. SUBCHAPTER E—ANIMAL DRUGS, FEEDS, AND RELATED .507 Agency Food and Drug Administration, Department of Health and Human Services.[CITE: 21CFR507] TITLE 21--FOOD AND DRUGS.2 (e) of this chapter.12) Subpart B—Current Good Manufacturing Practice (§§ 507.28) Subpart C—Hazard Analysis and Risk-Based Preventive Controls (§§ 507. Use the navigation links in the gray bar above to view the table of contents that this content belongs to.

The in-page Table of Contents is available only when multiple sections are being viewed. 262) and § 601. This online reference for CFR Title 21 is updated once a year.View the PDF for 21 CFR Part 507 Subpart F; These links go to the official, published CFR, which is updated annually.12, if a facility is required to comply with subpart B of part 507 and is also required to comply with subpart B of part 117 of this .200 - Records subject to the requirements of this subpart.12, if a facility is required to comply with subpart B of part 507 and is also required to comply with subpart B of part 117 of this chapter because the facility manufactures, processes, packs, or holds .govCFR - Code of Federal Regulations Title 21 - Food and . As a result, it may not include . Title 21 was last amended 4/11/2024. ( b) Except as provided in § 56.

As a result, it may not include the most recent .A copy shall be given to the person signing the form.