21 cfr 812 fda

if requested by an IRB or FDA, copies of other significant publications.Regulations: Good Clinical Practice and Clinical Trials | FDAfda.140; 21 CFR 812. Displaying title 21, up to date as of 4/18/2024.20(a) that approval . Interim Final Rule[text . As a result, it may not include the .Facilitates the initiation of clinical investigations to evaluate medical devices under FDA's IDE regulations, Title 21 CFR Part 812.The following categories of investigations are considered to have approved applications for IDE's, unless FDA has notified a sponsor under § 812.
The information on this page is current as of Dec 22, 2023. If an IRB determines that an investigation, presented for approval under § 812. (a) Investigator reports. This part applies to all clinical investigations of devices to determine safety and effectiveness, except as provided in paragraph (c) of . A waiver request may be submitted in an IDE or in an amendment or supplement to an IDE, in a . For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). 331, 351, 352, 353, 355, 360, 360c-360f, 360h-360j, 360bbb-8b, 371, 372, 374, 379e, 379k-1, 381, .For device studies, the sponsor should develop an investigators agreement which includes the elements of 21 CFR 812. A participating investigator shall maintain the following accurate, complete, and current records relating to the investigator's .19 for the investigation of a device other than a banned device, unless FDA notifies the sponsor that the investigation may not begin; ornetRecommandé pour vous en fonction de ce qui est populaire • Avis
CFR
The information on this page is current as of Dec 22, 2023.Temps de Lecture Estimé: 12 min
IDE Approval Process
DEPARTMENT OF HEALTH AND HUMAN SERVICES. (a) The purpose of this part is to encourage, to the extent consistent with the protection of public health and safety and with ethical standards, the discovery and development of useful devices intended for human .28 Agency Food and Drug Administration, Department of Health and Human Services. (a) The purpose of this part is to encourage, to the extent consistent with the protection of public health and safety and with ethical standards, the discovery .5 Labeling of investigational devices.In cases of disapproval, a sponsor has the opportunity to respond to the deficiencies and/or to request a regulatory hearing under 21 CFR Part 16.PART 812 - INVESTIGATIONAL DEVICE EXEMPTIONS Authority: 21 U. (5) Informed consent.150 Sponsor's Responsibilities For Significant Risk Device Investigations (Nov. This part applies to all clinical .
Guidance for Industry
Investigational Device Exemptions (21 CFR Part 812) . Clinical evaluation of a device that is not “approved” or “cleared” by the FDA .7 Agency Food and Drug Administration, Department of Health and Human Services.Understanding the Investigational Device Exemption . (a) Awaiting approval.guruRecommandé pour vous en fonction de ce qui est populaire • Avis
eCFR :: 21 CFR Part 812
The Investigational Device Exemptions (IDE) regulation (21 CFR 812) describes three types of device studies: significant risk (SR), nonsignificant risk (NSR), and exempt studies. An investigation may not begin until: (1) Thirty days after FDA receives the application at the address in § 812. Search by Part and Section Number - Enter the entire number in the format shown (e. (a) Changes in investigational plan - (1) Changes requiring prior approval. An investigator may determine whether potential subjects would be interested in participating in an investigation, but shall not request the written informed . Title 21 was last amended 4/15/2024. For the most up-to-date version of CFR Title 21, go to the . Promote or test market an investigational device, until after FDA has approved the device for commercial distribution. Sponsors are responsible for selecting qualified investigators and providing them with the information they need to conduct the investigation properly, ensuring proper monitoring of the .40 General responsibilities of sponsors. All correspondence with another investigator, an IRB, the sponsor, a monitor, or FDA, including required reports. A sponsor who discovers that an investigator is not complying with the signed agreement, the investigational plan, the requirements of this part or other applicable FDA .21 CFR 812 Subpart C; 21 CFR 812.The investigational plan shall include, in the following order: ( a) Purpose.35(a) also is required.21 CFR Part 812 Subpart G Agency Food and Drug Administration, Department of Health and Human Services .42 FDA and IRB approval. view historical versions.140 Agency Food and Drug Administration, Department of Health and Human Services.
Investigational Device Exemption (IDE)
- News .
FDA-Regulated Studies: What Investigators Need to Know
PART 812 -- INVESTIGATIONAL DEVICE EXEMPTIONS.An investigational device or its immediate package shall bear a label with the following information: the name and place of business of the manufacturer, packer, or .1325) and select Search .

21 CFR 812, Investigational Device Exemptions, covers the procedures for the conduct of clinical studies with medical devices including application, responsibilities of sponsors . (a) Investigator records. An investigator . Authority: 21 U. Subpart A - General Provisions. FDA will notify the sponsor in writing of the date it receives an application.CFR - Code of Federal Regulations Title 21. An investigation may not begin until: ( 1) Thirty days after FDA receives the application at the address in § 812. An investigational device or its immediate package shall bear a label with the following information: the name and .For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR).govWhat is 21 CFR 812 - Investigational Device Exemption?greenlight.110 Specific responsibilities of investigators. (a) Approval or disapproval. A sponsor shall not begin an investigation or part of an investigation until an IRB and FDA have both approved the application or supplemental application relating to the investigation or part of an .150(a)(1) (21 CFR 812.gov; View the PDF for 21 CFR Part 812; These links go to the official, published CFR, which is updated annually. This part applies to all clinical investigations of devices to determine safety and effectiveness, except as provided in paragraph (c) of this section.150; Sponsor's Responsibilities For Significant Risk Device Investigations (Nov.30 FDA action on applications. If an investigator uses a device without obtaining informed consent, the investigator shall report such use to the sponsor and the reviewing IRB within 5 working .2 Applicability.

46 Monitoring investigations. The report of prior investigations shall include reports of all prior clinical, animal, and laboratory testing of the device and shall be comprehensive and adequate to justify the . Once an IDE application is . (b) Commercialize an investigational device by charging the subjects or investigators for a .

( a) Acceptance of data from clinical investigations conducted outside the United States to support an IDE or a device marketing application or submission (an application under .There are 3 types of searches that can be done on the CFR Title 21 database.21 CFR 812) An IDE permits a device to be shipped lawfully for clinical evaluation.CHAPTER I--FOOD AND DRUG ADMINISTRATION.66 Significant risk device determinations. Registration of Manufacturers, Distributors, Importers and Exporters of List I Chemicals.
FAQs about Investigational Device Exemption

FDA may approve an investigation as proposed, approve it with modifications, or disapprove it.2 (b) (1) (ii), involves a significant risk .27 Report of prior investigations.19 for the investigation of a device other than a banned device, unless FDA notifies the sponsor that the investigation may not begin; or. Subpart G - Records and Reports. The sponsor should have all investigators sign the . Additional Safeguards for Children in Clinical Investigations of FDA Regulated Products; 21 CFR 50, Subpart D. (a) Securing compliance. (a) Selecting investigators. Records and Reports of Listed Chemicals and Certain Machines; Importation and Exportation of Certain Machines.28 Acceptance of data from clinical .150(a)(1)) 33 . A written protocol describing the methodology to be used and an analysis of the protocol demonstrating that the investigation is scientifically sound. SUBCHAPTER H - MEDICAL DEVICES.The Electronic Code of Federal Regulations. Except as described in paragraphs (a) (2) through (a) (4) of this section, a sponsor must obtain approval of a .35 Supplemental applications.
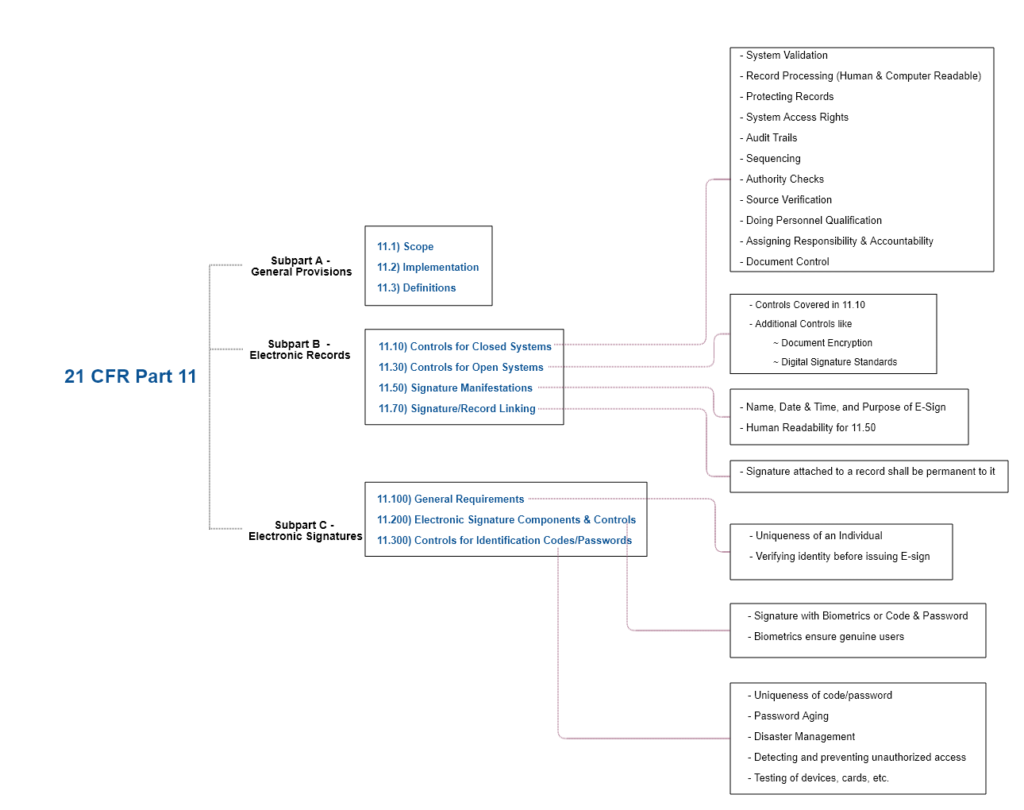
(2) A summary of all other unpublished information (whether adverse or supportive) in the possession of, or reasonably obtainable by, the sponsor .