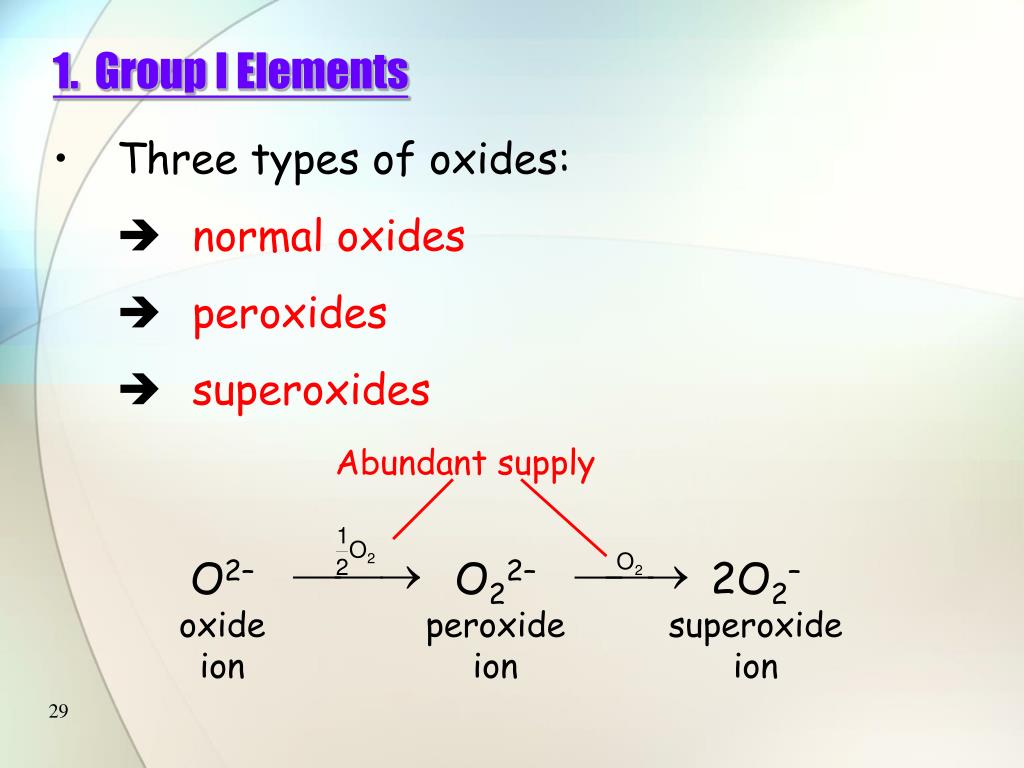
3 types of oxides
Oxides are compounds made from one or more atoms of oxygen combined with one other element. Oxides are binary compounds of oxygen with another element, .Oxides are chemical compounds with one or more oxygen atoms combined with another element (e.comRecommandé pour vous en fonction de ce qui est populaire • Avis
Oxides-Classification And Periodic Trends Of Oxides
Examples of acidic oxides phosphoric acid, H 3 PO 4 P 4 O 10 phosphorous(V) oxide CO 2 SO 2 SO 3 Formula carbonic acid, H 2 CO 3 sulphurous acid, H 2 SO 3 sulphuric acid, H 2 SO 4 Acid Produced with Water carbon dioxide sulphur dioxide sulphur trioxide Acidic Oxide Find out more with this guide for KS3 physics students aged 11-14 from BBC Bitesize. Mixed metal oxides: mixed metal . They can react with acids and, in a few cases, with bases. Studied by 0 people. Calcium Oxide CaO. Green and reddish brown stains on a limestone core sample, respectively corresponding to oxides/hydroxides of Fe 2+ and Fe 3+.This page explains the relationship between the physical properties of the oxides of Period 3 elements (sodium to chlorine) and their structures. Na 2 O, CaO, Al 2 O 3, CO 2, NO 2, Cl .
The reducing property decreases from $$\mathrm{H}_2 \mathrm{~S}$$ to $$\mathrm{H}_2$$ Te down the group. Full size table.Binary compounds of oxygen with elements of the periodic table are called oxides. There are different types of pottery oxides that artists can utilize to achieve specific results in their work.4K views 3 years ago.
What Are Pottery Oxides And How Are They Used

There are three main types of oxides: Acid oxides.Stoichiometries Iron oxide pigment. Simple Metal Oxides: A simple metal oxide is one carrying several oxygen atoms that the normal valency of its metal allows. Acid oxides contain more oxygen atoms than they do atoms of the element they .Regarder la vidéo18:05Properties and reactions of acidic, basic, amphoteric and neutral oxidesAuteur : Dr Hanaa Assil - Chemistry TeacherA summary of the potential uses of Mn oxides for soil remediation, as well as the type of Mn oxides and the maturity of each technique are listed in Table 7. Introduction to metal and non-metal oxides. Classifying Oxides. Amphoteric oxides are classified as metal oxides that react with both acids as well as bases to create salts and water.The number of different structures and compositions that can be generated for oxides is almost limitless. However, the development . B It reacts with an acid to form a salt. $$\mathrm{TeO}_2$$ is an oxidising agent while $$\mathrm{SO}_2$$ is reducing in nature.Memorize terms like List 3 examples of Basic Oxides , What is the bonding of basic oxides? , List 3 examples of Acidic Oxides and others.5 Conclusion and Future Perspectives. Simple Oxides: Have you ever considered how simple and complex an oxide can . Search for anything. Definition of oxides. Truro School in Cornwall. AMPHOTERIC OXIDES.Any chemical compound that contains O 2- as its anion is also termed an oxide. Overall, oxides of transition metals with the lowest oxidation states are basic (and react with acids), the intermediate ones are amphoteric, and the highest oxidation states are primarily acidic.Physical Properties of Period 3 Oxides. Examples of oxides include: MgO, ZnO, K 2 O, CO 2, SO 2, H 2 O. Basic Oxides, also known as Metal Oxides, are typically formed when metals react with oxygen.An oxide is an ion of oxygen with oxidation state equal to -2 or O 2-., CO 2, SO 2, CaO, CO, ZnO, BaO 2, H 2 O, etc.In general, if the volume of the metal oxide per mole of metal is greater than the molar volume of the metal, the oxide will form a protective scale. The ozone molecule contains five lone pairs of electrons. Home Explore Exams Blog. Acidic and basic oxides have different properties and values of pH. Examples: CO, NO, H2O. The trend in acid-base behavior can be summarized as follows: Acidity increases from left to right, ranging from strongly basic oxides on the left to strongly acidic ones on the right, with an amphoteric oxide (aluminum oxide) in the middle.

The reactants may be elements or compounds, while the product is always a compound. Examples include MgO, CaO, CuO, ZnO, NiO, Al 2 O 3, Fe 3 O 4, BaO, etc.There are different properties which help distinguish between the three types of oxides. How does metal oxides protect a metal from further oxidation? How does oxidation rate progress on metal surfaces?
Oxides
2 – Types of Oxides.Synthesis Reactions.The oxides: The oxides in the top row are the highest known oxides of the various elements, in which the Period 3 elements are in their highest oxidation states. The O2- ion forms compounds with more than 90 elements in the . Discussion Questions. Examples: Magnesium reacts with oxygen to form magnesium oxide. Examples: Oxides formed with metals near ?STEPS? such as ZnO, Al2O3, PbO, Properties: 1.With the exception of CrO 3 and Mn 2 O 7, transition metal oxides are not soluble in water. Argon is obviously omitted because .
PHYSICAL PROPERTIES OF THE PERIOD 3 OXIDES
Analyzing the reactants and products of a given reaction will allow you to place it into one of these categories. For those taking GCE ‘O’ Level syllabus, there are many questions that will come out for this section on Types .Metal oxides, metal oxides nanoparticles, and mixed-metal oxides are a few emerging compounds that make up a vital class of substantial materials, having . A basic oxide . The main types include iron oxide, copper oxide, manganese oxide, cobalt oxide, and chrome oxide. Oxides are compounds of metals or non-metals with oxygen. In this video, we look at the four common types of oxides: basic oxides, acidic oxides, neutral oxides & amphoteric oxides.Both the types of oxides are acidic in nature. The oxide anion combines with cations with charges that range from +1 to +7.Oxides of all the metallic elements are known and they show a wide range of properties in terms of structures, acidity and basicity, and conductivity. Metal and non-metal oxides.Amphoteric Oxides - Amphoteric oxides are found in the lighter elements of Groups 2 and 13, some of the d-block elements, and the heavier elements of Groups 14 and 15. Login Get started. Simple Oxides: Acidic Oxide, Basic Oxide, and Amphoteric Oxide. The second group includes ionic or basic oxides, such as CaO and .Examples of Oxides – Al2O3 – Aluminum oxide, CO2 – Carbon dioxide, SO2 – Sulfur dioxide, CaO – Calcium oxide, MgO – Magnesium oxide, Na2O – .comWhat Is an Oxide? Definition and Examples - ThoughtCothoughtco.Types of Oxides Types of Oxides.Types of Pottery Oxides. Namely, an oxide can exhibit molecular, 1-dimensional chain, 2 . Other examples of metallic oxides – Na 2 O, Al 2 O 3, .com/IGCSE/ChemistryNada AK is lecturing us some serious . This page explains the relationship between the physical properties of the oxides of Period 3 . C It reacts with an ammonium salt to form ammonia.
are rather unusual types of oxides (4,5) Crossword Clue
The general equation for a synthesis reaction is: A + B → AB. Section 1: Basic Oxides. Part of Chemistry Periodic table. Neutral oxides. The O2- ion forms compounds with more than 90 elements in the Periodic Table that are capable of losing electrons to form cations. The exceptional sorption and degradation properties of natural Mn oxides . To learn more about Identification, Examples, FAQs with .Solid oxide electrolysis cells (SOECs) have attracted widespread attention because of their high Faraday efficiency and controllability. Examples of oxides. The brown color indicates that iron is at the oxidation state +3.The alkali metals and the alkaline earth metals, as well as the transition metals and the posttransition metals (in their lower oxidation states), form ionic oxides—i. Choose the correct answer from the .Metals and non-metals can be heated in oxygen to make compounds called oxides.
O Level Chemistry
The five basic types of chemical reactions are combination, decomposition, single-replacement, double-replacement, and combustion.0 (0)add a rating.
Sodium Oxide Na2O. Study with Knowt flashcards for free. The health impacts for children exposed to lead include behaviour and learning problems, lower IQ and hyperactivity, slowed growth, hearing problems, and anemia.NEUTRAL OXIDES.3, for example, the total number of electrons lost by aluminum is equal to the total number gained by oxygen: electrons lost = 4Alatoms × 3e − lost Alatom = 12e − lost. Classify oxides as either acidic or basic, related to the metallic and non-metallic character of the other element in the oxide. Un oxyde contenant une proportion d'oxygène moins élevée ou plus élevée qu'un oxyde normal est appelé respectivement sous-oxyde 3 ou . The same pattern is seen in all oxidation–reduction reactions: the number of electrons . Multiple Choice. Find clues for are rather unusual types of oxides (4,5) or most any crossword answer or clues for crossword answers.
An amphoteric oxide is one which shows both acidic and basic properties. Some people more loosely apply the term to refer to any compound where oxygen serves as the anion. The alkalinity of these solutions is . Search for crossword clues found in the Daily Celebrity, NY Times, Daily Mirror, Telegraph and major publications. Examples include calcium oxide, zinc oxide and magnesium oxide. The term anhydride (without water) refers to compounds that assimilate H 2 O to form either an acid or a base upon the addition of water.There are four classifications: acidic, basic, amphoteric, and neutral.Last Modified 25-01-2023. React with both acids and bases to form salt & water. Which property is characteristic of a base? not.Un oxyde 1, 2 est un composé de l' oxygène avec un élément moins électronégatif que lui, c'est-à-dire tous sauf le fluor a et lui-même. A synthesis reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. Le terme « oxyde » désigne également l' ion oxyde O 2− . Metal oxides are. Carbon dioxide is an acidic oxide that reacts with aqueous calcium hydroxide.


As we move across each period of the periodic table, the atomic number increases, as does the acidic nature .
Lesson Explainer: Reactions of Oxides
Lead poses health risks of particular concern for children and pregnant women.Answers for are rather unusual types of oxides (4,5) crossword clue, 9 letters. electrons gained = 6Oatoms × 2e − gained Oatom = 12e − gained. Metals and non-metals can react with oxygen to . Basic oxides dissolve in water to form alkaline solutions.Basic oxides, list, list, physical and chemical properties - . Acid and basic oxides. What is the bonding of basic oxides? get a hint. Oxides are binary compounds of oxygen with another element, e. They have the ability to react with acids, forming water and a type of salt, showcasing their basic properties. 2Mg + 2O 2 → 2MgO.