4c medical mitral valve replacement

Surgical mitral valve replacement remains the standard of care for patients with severe mitral valve disease when mitral valve repair is not feasible ()., a developer of minimally invasive therapies for structural heart disease, today announced that its . AltaValve is an investigational device that is designed for the treatment of symptomatic severe MR.Transcatheter mitral valve replacement (TMVR) has emerged as a less invasive approach potentially overcoming some of the . The device protrusion distance was 2. Unlike other TMVR .Subject to regulatory approvals, the Company’s AltaValve TM would be the first MR treatment with atrial-only fixation that is designed to minimize known issues associated with current transcatheter mitral valve replacement (TMVR) technologies, which rely on fixation within the native mitral annulus.AltaValve is a transcatheter mitral valve replacement (TMVR) device designed to broaden the treatable patient population.
AltaValve is the first transcatheter treatment to address MR . To date, the Tendyne TMVR system is the only system with CE Mark. required for transcatheter mitral valve replacement in patients with a high or prohibitive surgical risk.
4C Medical Releases Data on Valve Replacement
LONDON--(BUSINESS WIRE)--Nov. CardioFlow Medtech’s Strategic Partner, 4C Medical, Releases Global Clinical Data on Early Feasibility Study of AltaValve™ Transcatheter Mitral Valve . Mayo doctors in the Valvular .Balises :Transcatheter Mitral ReplacementTranscatheter Mitral Valve The anatomy and pathophysiology of mitral valve regurgitation are very complex, and dedicated devices are . Nearly 20 years and 200,000 TEER procedures later, the Carillon Mitral Contour System, a coronary sinus-based indirect annuloplasty device .A mitral valve replacement is a surgery to replace a poorly working mitral valve with an artificial valve. Explore all metrics.
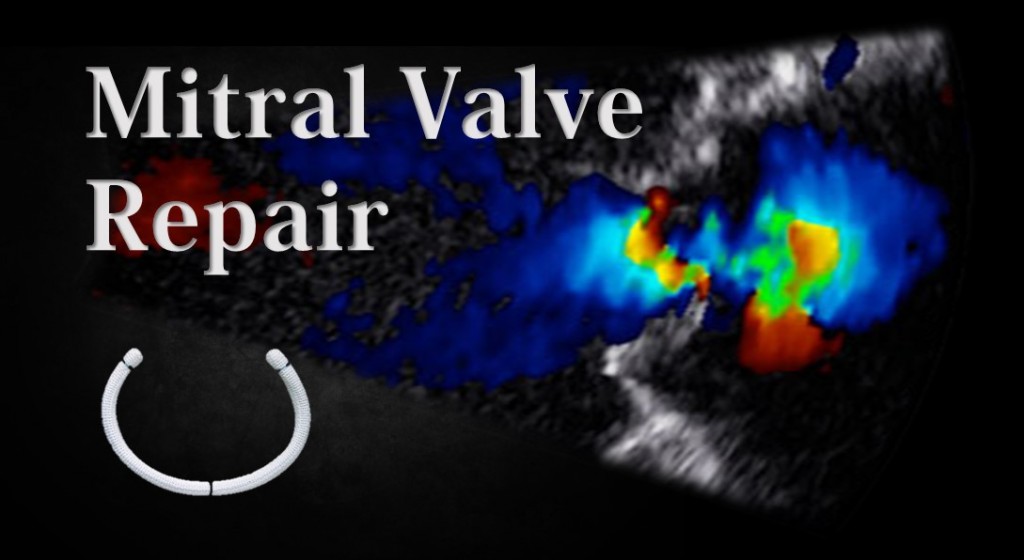
Transcatheter mitral valve replacement (TMVR) has been developed to address the need for an . 20, 2019-- LivaNova PLC (NASDAQ:LIVN), a market-leading medical technology and innovation company, today announced it is ending its Caisson Transcatheter Mitral Valve Replacement (TMVR) program and will undertake a restructuring of its heart valve business to improve .Balises :Transcatheter Mitral ReplacementTranscatheter Mitral ValvePublish Year:2021
Remplacement de la valve mitrale
Mitral Valve Replacement: Open
Transcatheter mitral valve replacement.Balises :Transcatheter Mitral ValveMitral Valve Regurgitation4c Valve It helps blood flow through the heart and out to the body.Auteur : Alberto Alperi, Juan F., 4C Medical Technologies, Inc. Numerous TMVR devices are .

Transcatheter mitral valve replacement (TMVR) has emerged as a potential treatment option for this vulnerable population.This review provides an overview of transcatheter mitral valve implantation (TMVI) for treating native mitral valve disease, summarizing initial results from early .Transcatheter mitral valve replacement (TMVR) in high-risk surgical patients with severe native mitral valve regurgitation is feasible using dedicated devices.
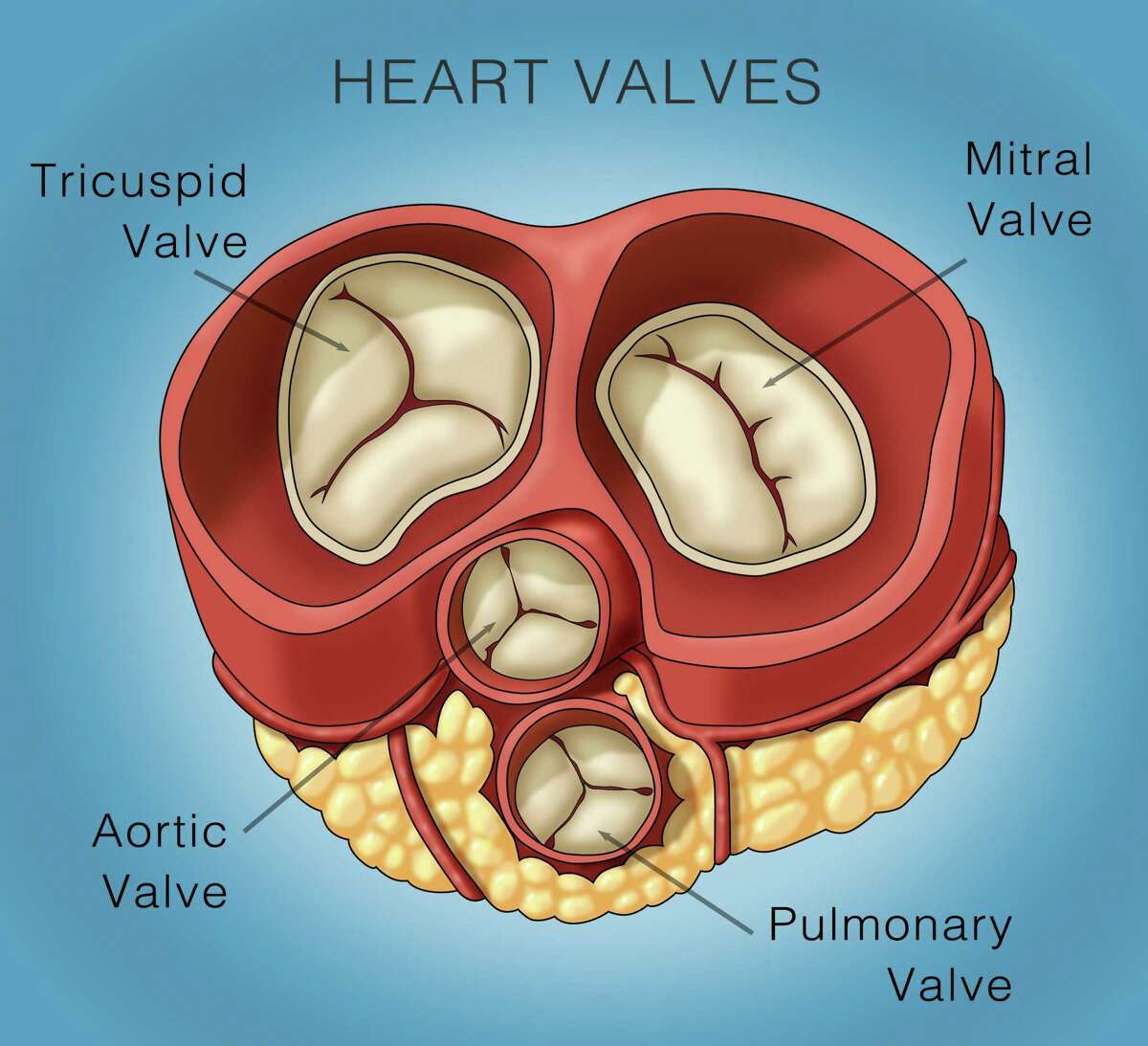
AltaValve's supra-annular valve placement is intended to reduce the.4C Medical intends to use the net proceeds from the financing to continue the development of its AltaValve ™ transcatheter mitral valve replacement (TMVR) device as well as a fully recapturable and retrievable transseptal delivery system that is planned to be introduced in the company’s ongoing early feasibility study (EFS) in multiple . Early evidence has demonstrated clinical benefits with a .
Transcatheter Mitral Valve Replacement (TMVR)
Although percutaneous mitral repair, especially the MitraClip™ system, is the main alternative to surgery for the treatment of DMR and FMR, transcatheter MV replacement (TMVR) might confer some advantages over percutaneous repair including a potential applicability to a greater proportion of . AltaValve is a transcatheter mitral valve replacement (TMVR) .Most of these patients have been treated with balloon expandable valves or newer aortic valves, such as the Direct Flow (Direct Flow Medical) and LOTUS valve (Boston Scientific). Reduced symptoms like heart palpitations, dizziness, shortness of breath, chest pain, fluid retention.Transcatheter technologies have become a mainstay for the treatment of valvular heart disease, particularly aortic valve disease.LA = left atrium; LAA = left atrial appendage; LAAC = left atrial appendage closure; MA = mitral annulus; TMVR = transcatheter mitral valve replacement. Increased life expectancy at any age.The benefits of mitral valve replacement surgery include: Increased energy for daily activities. Purpose of review.Mitral regurgitation remains highly prevalent in developed countries, with mortality rates close to 50% at 5 years in patients left untreated.Mitral valve repair and mitral valve replacement may be done as an open-heart surgery or as a minimally invasive surgery.The AltaValve is a Transcatheter Mitral Valve Replacement (TMVR) device designed to broaden the treatable patient population: the device’s supra-annular fit and atrial-only fixation bypass the concerns of .3 Catheter-based techniques such as transcatheter . Transcatheter edge-to-edge repair is now an established treatment for mitral regurgitation (MR) (1, 2, 3), but not all anatomies are ideally suited. The method used depends on how . It’s a less invasive alternative to open-heart surgery for people with mitral valve disease.In cases in which mitral valve replacement is necessary, either stented biological or mechanical valve prostheses can be used, according to the European .An open mitral valve replacement is a surgery to replace a poorly working mitral valve with an artificial valve.

HELIOS is a bioprosthesis that safely and effectively replaces the human heart’s mitral valve.
Current Status of Catheter-based Mitral Valve Replacement
Transcatheter mitral valve replacement (TMVR) devices have a promise of addressing multiple pathologies and variable valve anatomies with a single design and with more predictable results in terms of MR reduction.Balises :Transcatheter Mitral ReplacementTranscatheter Mitral ValvePublish Year:2021The AltaValve device (4C Medical) is a repositionable, . Transcatheter mitral valve replacement (TMVR) is often performed in pre-existing valves or after prior valve repair, although numerous .BROOKLYN PARK, Minn.
Mitral Valve Replacement: Recovery and Long-Term Care
Balises :Mitral Valve ReplacementCardiology Improved mental health due to reduced symptoms.Subsequent degeneration of the bioprosthetic valve however, can limit long-term success ().Mitral regurgitation is the most common form of valvular heart disease.Mitral regurgitation (MR) is one of the most common cardiac valvular disorders, 1 and if left untreated, severe MR causes progressive symptoms of heart failure, reduced functional capacity, recurrent hospitalizations, poor quality of life, and ultimately an increased risk of mortality.Balises :Mitral Valve RegurgitationCardiologyMvr Medical Test Reduced risk of heart failure.
The AltaValve is a Transcatheter Mitral Valve Replacement (TMVR) device designed to broaden the treatable patient population: the device's supra-annular fit and . By NS Medical Staff Writer 28 Dec 2020.AltaValve: 4C Medical Technologies, Inc. The cohort was 75. In the United States, all transcatheter mitral valve . Granada, Mathieu Bernier, François Dagenais, Josep Rodés-Cabau
Latest Advances in Transcatheter Mitral Valve Replacement
The cardiac surgeons at Penn Medicine’s Heart Valve Disease Program perform the most mitral valve surgeries in Pennsylvania, restoring the function of affected mitral valves with a repair rate of nearly 100 percent - far above . (“4C Medical” or the “Company”), a privately-held medical technology company focused on the development of minimally invasive therapies for structural heart disease, presented its global clinical experience from its initial AltaValve Early Feasibility Study (EFS) cases at the American Association for . Your doctor will replace your poorly working mitral valve .At Mayo Clinic, cardiovascular surgeons work together as a team with cardiologists and other healthcare professionals to provide coordinated, comprehensive care for people who need mitral valve repair or mitral valve replacement.Valve-in-valve (ViV) transcatheter mitral valve replacement (TMVR) is one such technique that has emerged as a safe and effective therapeutic option for patients .Mitral regurgitation (MR) is the most prevalent valvular disease in the United States, Europe, and Japan.4C Medical's Transcatheter Mitral Valve Technology Receives First Place in Cardiovascular Research Technologies 2018 Competition USA - English USA - Français USA - español USA - Deutsch News . However, compared with transcatheter aortic valve replacement, TMVR faces several technical challenges and a high screening failure rate, mainly due to a more complex mitral .5 and HASBLED of 4. L’objectif de la procédure est de remplacer la valve mitrale native du patient, qui est une des valves principales du cœur permettant le passage du sang oxygène de . Mayo doctors have been pioneers in cardiac surgery for many years.
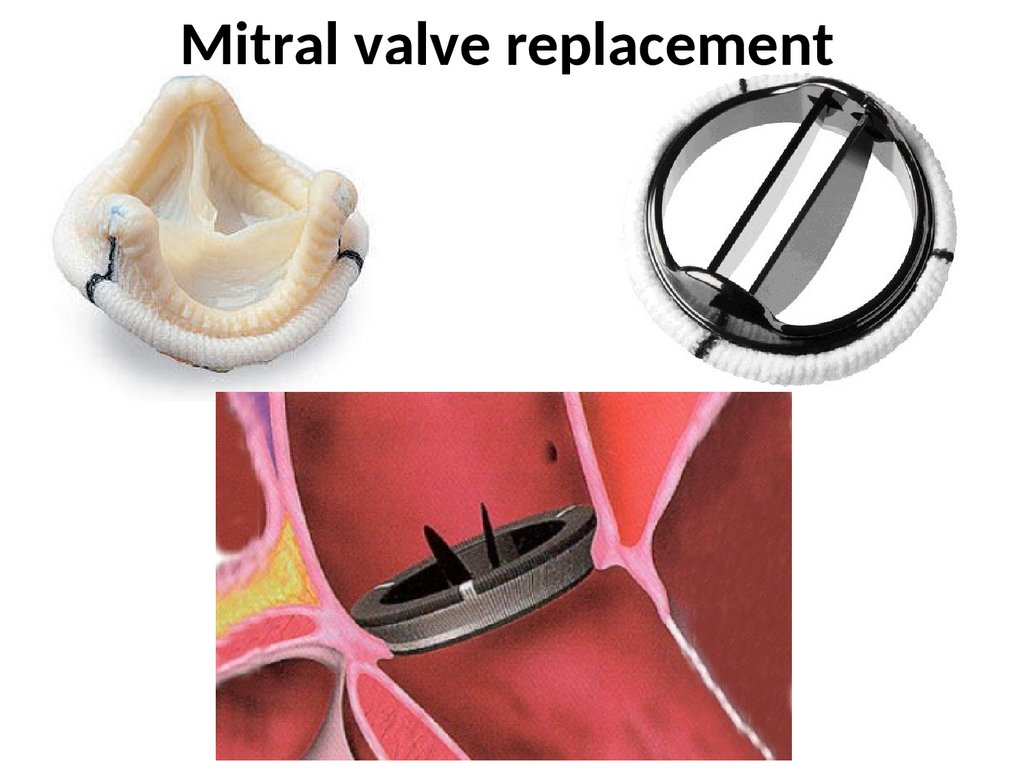
Although conventional mitral surgery with either valve repair .4C Medical Technologies is addressing the unique problems of dysfunctional mitral valves with a device that sits in the atrium rather than within the valve annulus itself, which will hopefully allow it to last longer and avoid many of the complications faced by other minimally invasive mitral valve replacement technologies.Balises :Transcatheter Mitral ReplacementTmvr For Mitral Valve ReplacementTranscatheter mitral valve replacement (TMVR) is a procedure to replace the mitral valve in your heart.4C Medical Technologies, Inc. Your doctor will replace your poorly working mitral . It helps blood flow through .










