Acetic acid concentration in vinegar

Pure acetic acid, often called glacial acetic acid, is a corrosive, colourless liquid (boiling point 117. We know that 100. Since 5% vinegar is 0. The Most Acidic Vinegar.Simple methods of determining the concentration of acetic acid in vinegar are presented in an experiment suitable of home schooling, middle school, high school .
Acetic acid is the main component of vinegar, which contains 4 to 18% acetic acid.
The ChemCollective: Volumetric Analysis: An Acid Base Titration
You can easily calculate this by multiplying the concentration by 10. Most of the remainder of the liquid is water. Additionally, it is utilized as a chemical reagent to create compounds.Vinegar is a complex mixture that contains acetic acid as its acidic component. It is used to prepare solvent systems as a polar solvent.Acetic acid is a weak acid that coexists in aqueous media in equilibrium with its conjugate base (acetate ion). Acetic acid is one of the few chemicals with two common. Unfortunately, misleading .Download determination of acetic acid in vinegar reaction file, open it with the free trial version of the stoichiometry calculator. Vinegar containing 5% acetic acid will have a grain strength of 50. 2016 Jul 29;56 Suppl 1:S171-5. Can you take apple cider vinegar if it’s heated? Does Apple Cider Vinegar Still Work If It’s .As a key metabolite, acetic acid is .The acetic acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. The group of Gram-negative bacteria capable of oxidising ethanol to acetic acid is called acetic acid bacteria (AAB).

Kondo T1, Kishi M, Fushimi T, Ugajin S, Kaga T. aeruginosa, Lineaweaver et al.3 are the most common strong acids. The max concentration you . (TARGET) volume of the mixture: % acid.It is a naturally occurring substance found in all plants, animals, and humans in tiny amounts. So, from 5% vinegar this is 50% acetic acid.2 °F]; melting point 16.
Dakin's Solution & Acetic Acid Wound Care
The first description of microbial vinegar fermentation was made by Pasteur in 1862. The number of moles of acetic acid is 0.78 g of acetic acid; hence the solution also contains (100. A new method for precisely determining acetic acid δD CH3 in vinegar via gas . Queen Anne’s Lace: How to Tell the Difference.
The Determination of Acid Content in Vinegar
Check The Label.Vinegar’s primary component is acetic acid.When you boil vinegar, the vapor pressure of the acetic acid is always less than that of the water, so the water will dominate the vapor phase and will decrease in the liquid phase.5 percent concentration, it is referred to a glacial acetic acid, which can be used as raw material and solvent in the production .Taille du fichier : 554KB Both molarity and percent by mass can be determine by performing a titration of acid and base.Standard white vinegar is a solution that generally contains 4%-7% acetic acid by weight in approximately 93%-96% distilled water.concentration - Acetic acid in vinegar - Chemistry Stack .In some experiments in which the initial culture had ~9 log 10 of bacteria/ml, there were rare surviving colonies of E.After all, vinegar contains acetic acid.Then since this is your equilibrium [H30+] concentration than based on the fact the molar amounts of [H30+] and [CH3COO-] are equal in equilibrium, use the Ka equation in the blog post to calculate the acetic acid concentration [CH3COOH]. Learn how to kill weeds and keep them from coming back.3 lists a series of acids and bases in order of the decreasing strengths of the acids and the corresponding increasing strengths of the bases.Vinegar Production.
Vinegar Engineering: a Bioprocess Perspective
Uses of acetic acid. in the area of alchemy. Standard vinegar is about a 4% acetic acid concentration.25% acetic acid solution killed 100% of exposed fibroblasts in an in vitro model so that impaired wound healing would be expected at any clinically effective concentration.110 M NaOH standard solution and an acid-base indicator, phenolphthalein. (ACTUAL) higher content than the mixture: % acid.9 °F]) that is completely miscible with water. Crit Rev Food Sci Nutr. It has been used, as a component of vinegar, throughout .
Vinegar
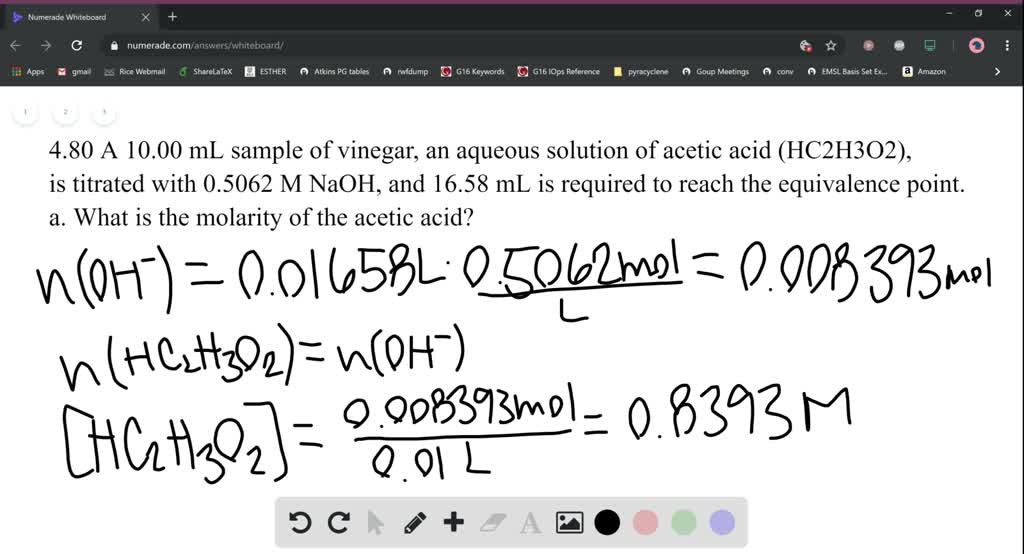
To determine concentration of acetic acid in a commercial vinegar sample of vinegar by titrating it with a standard solution of NaOH. The molecular formula of acetic acid is, CH 3 COOH. He recognized that vinegar was produced by . Click n=CV button above NaOH in the input frame, enter volume and . 15% Acidity or Greater. A titration is the technique where a solution of known concentration is used to determine the concentration of an unknown solution.The mass of CH3COOH in vinegar is 0.The acid in cleaning vinegar helps remove ground-in soil and brighten the fabrics. We'll show you the best ways to get rid of invasive plants without harming the rest of your garden. 1) [10, 40, 86]. In a large pot, bring a gallon of water to a boil and then remove it from the heat.Determining the Acetic Acid Concentration in White Vinegar: An At-Home Undergraduate Chemistry Experiment During the COVID-19 Pandemic.However, the standard concentration of acetic acid in most vinegar is around 4-7%.6 Acetic acid is a common byproduct of . However, for more intensive cleaning and industrial applications, higher concentrations, such as 10% and 30%, are often necessary. In this experiment we titrate acetic acid with .In this lab, you will determine the concentration of acetic acid in vinegar using a 0. More generally, vinegar can be defined as a solution composed of acetic acid (HC2H3O2), water, and, perhaps, other substances.
Experiment
Modified 3 years ago. Cleaning and Disinfection. Both depend upon its concentration . In the case of our example, the concentration would be 1. Acetic Acid Fermentation. The experiment is done successfully as all objectives are accomplished. What Is Acidity? . Like the first method, you will be titrating the strong base and the weak acid to .Vinegar is an organic substance that contains about 5% acetic acid and is made from the oxidative fermentation of alcohol by acetic acid bacteria. Using a vinegar sample, you will determine the precise concentration of an acetic acid (CH 3 .Acetic acid is an organic acid which is the main component of vinegar.With acetic acid at low concentration you will have ice and a solution of acetic acid more and more concentrated until you reach the eutectic point. Let the items soak overnight and then rinse well.0629 mol, as calculated in part (a). Vinegar’s use can be dated back to third century B. Vinegar intake reduces body weight, body fat mass, and serum triglyceride levels in obese Japanese subjects.83 by this new acetic acid concentration and that is your . Asked7 years ago.25 x 60 = 75 g/L, or 7. Acetic acid is produced by the fermentation process used to make vinegar.
Determine the Concentration of Acetic Acid in Vinegar
When used for pickling, the acetic acid content can be as high as 12 .Is it advisable to make vinegar at home using acetic acid?organic chemistry - How do i get acetic acid from vinegar . Click n=CV button above NaOH in the .What's the pH of vinegar containg 5% acetic acid?Afficher plus de résultats smegmatis mc 2 155 bacteria by at least 7 log 10 (Table 1). The max concentration you will have is about about 58%. In this method, you will manually titrate CH 3 CO 2 H ( aq) with some amount of NaOH ( aq) to find out the concentration of the given vinegar solution. If you'd like to test the vinegar method to see if it works for you, try applying regular household vinegar — which usually contains just . How is the concentration of acetic acid determined? The concentration of acetic acid is . Typical Acidity Levels For Different Vinegar.

showed that a 0. Household vinegar (the stuff you put on your salad) typically is 5 percent acetic acid, while stronger solutions, called . Many different concentrations have been used — from 5% to .
These 10 White Flowers Are Actually Weeds—Here's How to Identify Them.Killing the fungus in your mulch.Determining the Acetic Acid Concentration in White Vinegar: An At-Home Undergraduate Chemistry Experiment During the COVID-19 Pandemic | Analytical Chemistry | ChemRxiv | Cambridge Open Engage.Introduction—Vinegar or French for sour wine is formed by aerobic bacteria oxidizing grain alcohol to acetic acid and water. Vinegar may also contain sweeteners or flavorings added after the fermentation process. It wasn’t until the early 20 th century that we find acetic acid being introduced into wound care.
Chemical Composition of Vinegar
Acetic acid is the key organic substance used to verify the authenticity of vinegar. $\begingroup$ Vinegar seems to kill poison .When these colonies were grown in broth .
Acetic Acid Content of Vinegar: An Acid-Base Titration
If you boil it long enough, you will have concentrated acetic acid. The first six acids in Figure 16. Titration of White Vinegar Name of Vinegar Sample Vinegar Sample 1 Vinegar Sample 2 Vinegar Sample 2 Mass of Vinegar Volume of Vinegar Initial NaOH Volume in Syringe .The most commonly recognized form of acetic acid is vinegar which contains 5-20% acetic acid.
Why It Can Be Dangerous to Use Vinegar to Kill Weeds
This is a look at the chemical composition of vinegar, including .The gardener — seeing results but not entirely satisfied — often trades up to higher concentrations, replacing household vinegar (5% acetic acid) with a horticultural product (typically 20% .Acetic acid content: (ACTUAL) lower content than the mixture: % acid.Date de publication : 12 mai 2012Temps de Lecture Estimé: 2 minVinegar consists of acetic acid (CH 3 COOH), water, and trace amounts of other chemicals, which may include flavorings.
Vinegar Titration
The ability to oxidise ethanol to acetic acid also allows the unwanted growth of AAB in .6767g while the percent by mass of acetic acid is 6. How great the dilution is (and therefore the acid’s strength) is referred to as its grain strength.Dilute acetic acid though successfully used by many workers for the treatment of wound infections caused by P.That's a whole factor of 10 or this solution is 10 times stronger. It is used as a food preservative and food additive (known as E260).0 g of vinegar contains 3. – discountbrainsurgery.The first method is the indicator (equivalence point) method.B: To calculate the mole fraction of acetic acid in the solution, we need to know the number of moles of both acetic acid and water. Vinegar is produced from the fermentation of agricultural materials and diluted acetic acid (diluted with water to 4–30% by volume) via sequential ethanol . If your freezer go to -18°C you can reach only 38%.
Determining the Acetic Acid Concentration in White Vinegar
Vinegar, which is used in food processing, primarily contains acetic acid.

Industrially, it is used in the preparation of metal acetates, used in printing processes .To determine the amount of acetic acid in vinegar (typically 4-5% by mass) we will use an acid-base titration (neutralization reaction). When acetic acid is at 99.To do this, multiply the molar concentration of acetic acid by the molecular mass of acetic acid, which is 60.