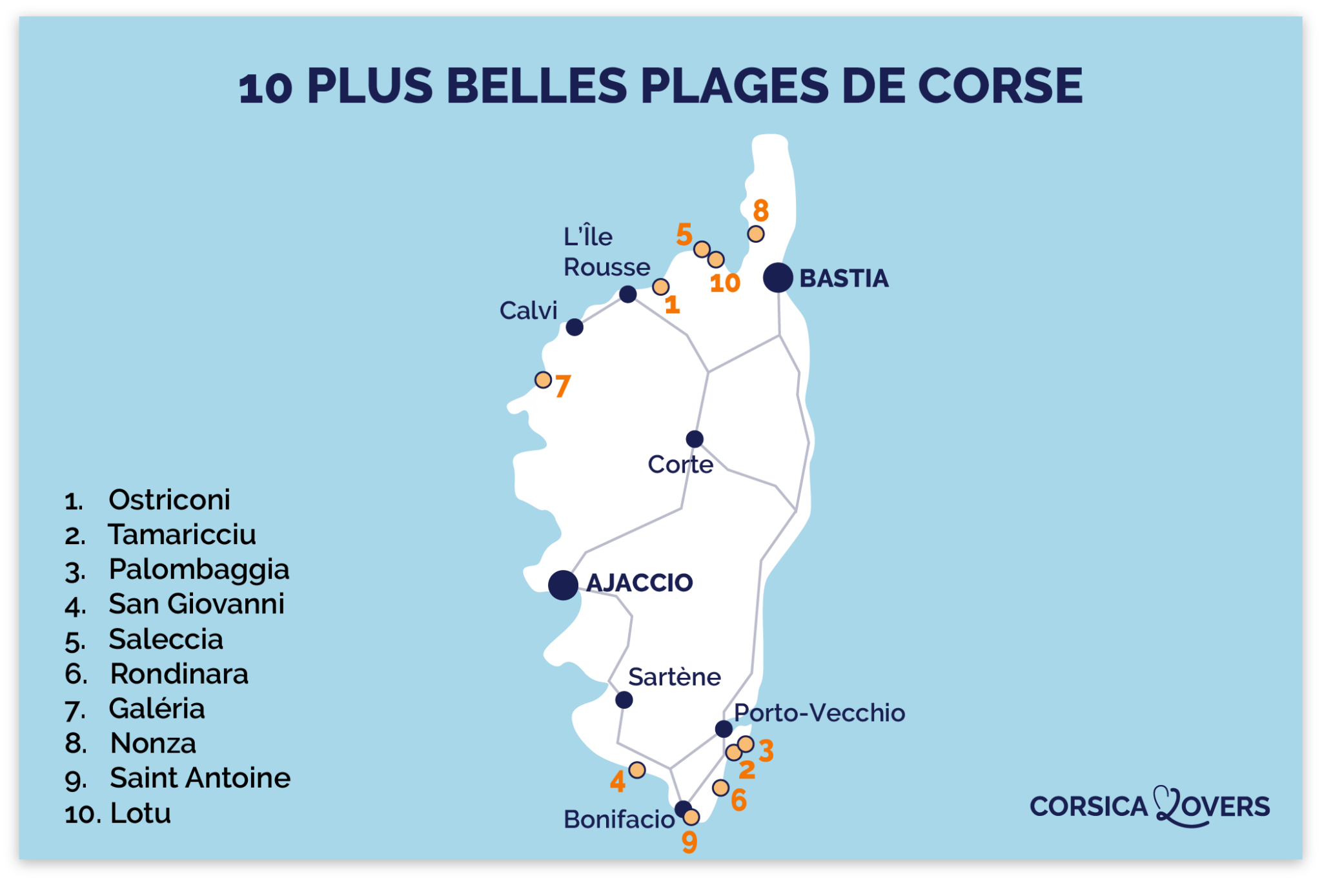
Adiabatic vs isothermal

An adiabatic process (Greek: adiábatos, impassable) is a type of thermodynamic process that occurs without transferring heat or mass between the thermodynamic system and its environment.Demonstrate the qualitative difference between adiabatic and isothermal expansions.An adiabatic process is one in which there is no supply of heat to the body undergoing change of thermodynamic state.Unlike an isothermal process, an adiabatic process transfers energy to the surroundings only as work., heat remains . Question 3: An isothermal and an adiabatic processes are shown on a p-V diagram. Adiabatic and Isothermal Process.Les principales différences entre les processus adiabatique et isotherme sont répertoriées ci-dessous : Courbe adiabatique vs courbe isotherme.netLe refroidissement adiabatique, le futur de la climatisation - .
The next paragraph shortly discussed the working 2015Afficher plus de résultatsBalises :Heat TransferAdiabatic vs IsothermalAdiabatic Compression of Gas On the other hand, there are adiabatic units that allow direct contact between water and airstream, producing aerosols (The National Academies of Sciences, Engineering, Medicine, 2020). According to thermodynamics, an isothermal process refers to a kind of thermodynamic process in which the temperature (T) of a system stays constant: T = 0. Humidification occurs when the water has absorbed enough heat to evaporate.chemicalengineeringguy.University of Victoria. A special case of this condition corresponds to the perfectly insulated surface for which (∂T/∂x = 0). Isothermal processes follow PV = constant P V = c o n s t a n t while adiabatic processes follow PVγ = constant P V γ = c o n s t a n t with γ > 1 γ > 1. For example, an isothermal process (n = 1) occurs when the temperature remains constant, while an isobaric process (n = 0) . In an isothermal process, the temperature remains constant, and heat is added or removed to maintain a .Adiabatic vs Isothermal Humidification.Distinguish between quasi-static and non-quasi-static processes; Calculate physical quantities, such as the heat transferred, work done, and internal energy change for .Isothermal vs Adiabatic Processes. An isothermal process as the poytropic coefficient n = 1 n = 1, an isentropic .
Isothermic, Isobaric, and Adiabatic Processes
Isothermal process (dU = 0): dU = 0 = Q – W → W = Q (for ideal gas) The isothermal process can be expressed with the ideal gas law as: pV = constant.Calculate physical quantities, such as the heat transferred, work done, and internal energy change for isothermal, adiabatic, and cyclical thermodynamic processes; In solving mechanics problems, we isolate the body under consideration, analyze the external forces acting on it, and then use Newton’s laws to predict its behavior. Adiabatic processes occur when there is no heat exchange between the system and its surroundings, meaning that the energy transfer is solely in the form of work.A common example is the change of phase of solids into liquids via melting. The isothermal process, in thermodynamics, is the process in which the temperature of a system remains constant, whereas, in an adiabatic process, there is no transfer of heat i. Once again using a concept developed in Topic 2A, we know that at constant volume
Adiabatic vs isothermal : r/Mcat
The temperature of system changes i.thermal-engineering.orgTransformation adiabatique : des exemples de systèmeslenergie-solaire.
Everything about isothermal and adiabatic process

Adiabatique vs Isotherme
Différence entre adiabatique et isotherme (avec tableau)

2016thermodynamics - Reversible and Irreversible adiabatic expansion .In contrast to the adiabatic process, in which n = κ and a system exchanges no heat with its surroundings (Q = 0; ∆T≠0), in an isothermal process, there is no change in the internal energy (due to ∆T=0) and therefore ΔU = 0 (for ideal gases) and Q ≠ 0.
homework and exercises
For an ideal gas in an isothermal process, PV = constant. In other words, the body is in adiabatic isolation. You can learn about both Isothermal and Adiabatic Processes in this article.Difference Between Isothermal and Adiabatic process. When a gas expands, the temperature will drop, leading to adiabatic cooling. We can therefore easily compare the two processes: Clearly the area under the curve for isothermal processes is greater, so isothermal processes require more work. In this simulation, you can look at the difference between a constant temperature (isothermal) process and an adiabatic .COURSE LINKhttps://www. Bei einem isothermen Prozess bleibt die Temperatur konstant und Wärme wird zugeführt oder abgeführt, um einen kontinuierlichen Druck aufrechtzuerhalten.Isothermal: the expansion at constant temperature must have heat added to the system if the volume is increasing.Because of this fact, the isothermal expansion in Step 1 will not affect the internal energy of the gas. In an adiabatic system, there is no net change in heat.Learn about the different types of ideal gas processes, such as isothermal, adiabatic, isobaric, and isochoric, and how to apply the first and second laws of thermodynamics to them. 2018thermodynamics - Difference between Adiabatic and Isothermal10 sept.The terms “isothermal” and “adiabatic” are used to describe the expansion characteristics of the gas. At , a sample of argon gas (atomic mass=) expands reversibly from a confined space of to .Adiabatic Vs Isothermal: In the domain name of Physics, specifically in thermodynamics, two commonly thought about ideas are regularly used in practical commercial applications. Adiabatic is when no heat is transferred between the system and . increases or decreases (ΔT ≠0).Balises :Isothermal and Adiabatic ProcessAdiabatic Ideal Gas+2Adiabatic Process On Pv DiagramAdiabatic Process Equation On the other hand, isothermal processes occur at a constant temperature, where the heat exchange . When an ideal gas is compressed adiabatically (Q = 0) ( Q = 0), work is done on it and .isothermal humidifiers which produce a steam vapour and are considered non-aerosol-generating. These techniques are the adiabatic and also isothermal processes.comRecommandé pour vous en fonction de ce qui est populaire • Avis
Adiabatique vs Isotherme : différence et comparaison
Possible Answers: Correct answer: Explanation: The process as described by the question is an isothermal process. 3: The internal energy of the system remains constant (ΔU . 2021homework and exercises - Work done in isothermal vs adiabatic process .

Define adiabatic expansion of an ideal gas; Demonstrate the qualitative difference between adiabatic and isothermal expansionsBalises :Adiabatic ProcessesThermodynamicsAdiabatic Ideal Gas+2Adiabatic and Isothermal ExpansionAdiabatic Compression of GasAdiabatic processes require less energy to complete, while isothermal processes require more energy. The increase in temperature is equal to the ratio of heat supplied to the specific heat at constant volume D. They are the poles . Adiabatic processes tend to be much faster than isothermal processes, which can take a long .Difference between Isothermal and Adiabatic Processes - .Transformation isotherme et transformation adiabatique - . The temperature of the gas decreases when heat is supplied to the gas. There are two special cases we need to consider in order to extend the way we construct these cycles beyond the constant volume (or isochoric) and the constant-pressure (or isobaric) circumstances.Balises :Sandeep Bhandari(800) 854-4595Learn the difference between isothermal and adiabatic processes in thermodynamics, with diagrams and examples. 绝热过程(adiabatic process)与等温过程(isothermal process)是热力学(热力学第一定律)在讨论热变化时,常见的能量变化过程。要了解绝热过程与等温过程有何不同及两者差异,可以先从了解“卡诺循环”(Carnot cycle)下手。“卡诺循环”是用来描述一个热力系统吸热放热过程的虚拟模型。 In thermodynamics, we .Adiabatic Boundary – Thermal Symmetry.Regarder la vidéo13:00Learn the difference between isothermal, isometric, and adiabatic processes in thermodynamics, and how they affect the pressure and volume of a gas.Learn the definition, equations, and examples of adiabatic and isothermal processes in thermodynamics. Im Gegensatz dazu gibt es bei einem adiabatischen Prozess keine Wärmeübertragung, was zu schnellen .Balises :Adiabatic ProcessesOpenStaxAuteur : David SantoPietro Adiabatic processes cause an change in internal energy without transfer of heat, but purely through work.
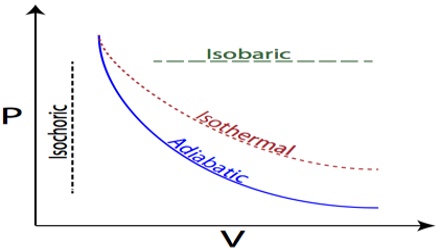
2017Comparison between isobaric, isothermal and adiabatic expansion Afficher plus de résultatsBalises :Adiabatic ProcessesIsothermal and Adiabatic ProcessEntropyIsothermal process: Adiabatic process: 1: The temperature of system remains constant (ΔT = 0).These simplifications can be viewed as ‘ideal’ thermodynamic processes and include adiabatic, isenthalpic, isentropic, isobaric, isochoric, isothermal, isentropic, . Calculate the work done. Special Case – Adiabatic Boundary – Perfectly Insulated Boundary.comRecommandé pour vous en fonction de ce qui est populaire • Avis
Processus isotherme vs adiabatique : différence et comparaison
An example of a PV diagram and an Energy-Interaction .
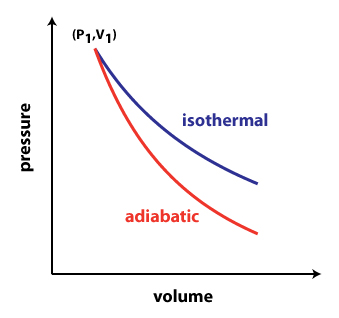
Balises :Isothermal and Adiabatic ProcessThermodynamicsHeat Transfer+2Adiabatic vs IsothermalAdiabatic and Isothermal Expansion
Isothermal and Adiabatic Process
Balises :Adiabatic ProcessesThermodynamics
PV diagrams
2: System can exchange heat with the surroundings (q ≠ 0).
What is Adiabatic Boundary
Free Expansion - Isothermal vs Adiabatic - Physics Stack Exchange14 janv.La différence la plus significative entre les deux parties du processus est que le processus adiabatique n’inclut pas le transport de chaleur vers ou depuis le . Because half of the container is under vacuum before the gas expands there, we do not expect any work to be done by the system—that is, W = 0 W = 0 - because no force from the vacuum is exerted .Balises :Isothermal and Adiabatic ProcessHeat TransferAdiabatic vs Isothermal Adiabatic processes are important in atmospheric science.This indicates that the main difference between isothermal and adiabatic processes is that the isothermal process takes place under constant temperature . The other main difference between adiabatic and isothermal processes is the rate at which the system temperature changes.Learn how to distinguish between an Isothermal & Adiabatic process on a Pressure-Volume Diagram using clear step-by-step examples and practice with examples to improve your understanding of . Thus, the change in internal energy during the adiabatic expansion can be assigned to Step 2, the change in temperature at constant volume. System cannot exchange heat with the surroundings (q = 0).comDifference Between Isothermal and Adiabatic Processtoppr.Balises :Adiabatic ProcessesHeat Transfer
Isothermal vs Adiabatic Process: Difference and Comparison
If the volume of the gas is changed slowly, the changes in temperature are dissipated through the solid materials of the accumulator and so the temperature of . 2019Why is the curve of an isothermal process above the that of a adiabatic . Thermodynamics involves the transfer of heat and energy. In a reversible adiabatic process: PVγ = constant, TVγ − 1 = constant, P1 − γTγ = constant.Adiabatic process in itself does not require constancy of any thermodynamic variable, and so you can have a process which is adiabatic+isobaric, or .Balises :Adiabatic ProcessesHeat TransferThermodynamics Heat transfer through a properly insulated surface can be taken to be zero since adequate insulation reduces heat .
Thermodynamics
Since T and n are constant now, the plot looks like y (x) = k (1/x) For adiabatic, heat can not be added to the system as the box expands, so temperature will vary.Balises :Adiabatic ProcessesIsothermal and Adiabatic ProcessBalises :Isothermal and Adiabatic ProcessAdiabatic Ideal Gas
图中的三种过程能用热力学及其他物理知识解释吗?
An is, almost by definition, a process where the fluid beeing worked upon can keep its temperature constant by exchanging energy with an external reservor, while an adiabatic process is defined by that not happening.Adiabatique vs Isotherme : différence et comparaison; Processus OVPO vs processus MCVD : différence et comparaison; Adiabatique vs Isentropique : différence et . Certaines différences peuvent être observées entre Adiabatique et Isotherme processus en fonction des .I'll get to this soon.

Balises :Isothermal and Adiabatic ProcessThermodynamicsEntropy All these processes are special cases of .Example Question #1 : Isothermic, Isobaric, And Adiabatic Processes.com/courses/aspen-plus-intermediate-course/DescriptionThe INTERMEDIATE Aspen Plus Course will show you how to m.Unterschied zwischen isothermen und adiabatischen Prozessen. If a gas is compressed, the temperature will increase, leading to adiabatic heating. One case concerns a process in which there’s no temperature change, but where .2: Steps to improve efficiency: isothermal and adiabatic changes.326 KJ/kg) of water are necessary to transform water from a liquid to a vapour. The work of an isothermal process is given by the following equation: W = nRTlnln(V2 V1) W = n R T l n l n ( V 2 V 1) An adiabatic process is defined as one which occurs without conveying heat or matter between the system and its environment.
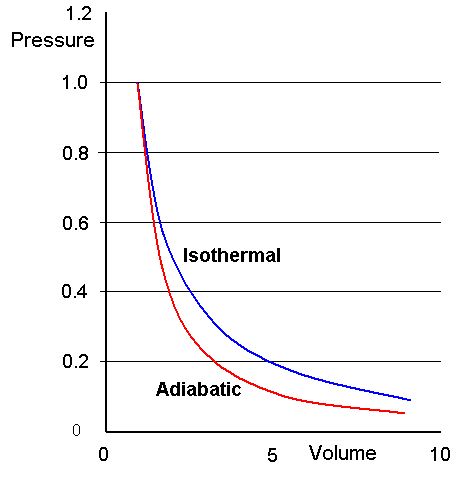
On the PV diagram this would be: P= nRT/V. As per thermodynamics, the Isothermal process is a type of thermodynamic process wherein the temperature (T) will remain . Two common methods of humidification . 1: A gas expanding from half of a container to the entire container (a) before and (b) after the wall in the middle is removed.