Ammonia bonding diagram

Balises :AmmoniaAtomsSolvolysisDiagramUSMLE Step 3 Valence electrons are the outermost electrons of an atom and are involved in bonding.Ammonia 1, a colorless, toxic gas, is an important industrial and agricultural chemical and a starting material for making a host of other compounds.Structure of ammonia.In the case of ammonia, the amount of hydrogen bonding is limited by the fact that each nitrogen only has one lone pair.The ammonia molecule is held together by the strong N–H nitrogen–hydrogen single covalent bonds by sharing electrons.
Covalent bonding
The new product is commonly known as the copper-ammonia complex ion, or more officially, hexamminecopper (II) complex ion. Covalent bonding results in the formation of molecules.Balises :AmmoniaNitrogenAtomsChemistryHydrogen Remember that each hydrogen atom should not have more than 2 electrons and the nitrogen atom should have a total of 8 electrons.GCSE; OCR 21st Century; Covalent bonds Dot and cross diagram. In the lewis structure of ammonia (NH 3), there are three N-H bonds and one lone pair on nitrogen atom.Balises :Nh3 BondNh3 Dot and Cross DiagramNFL Sunday TicketMoleculesammonia at relatively low costs. Above on the right two of the full 'dot and cross' electronic Lewis diagram for the covalent bonding in the ammonia molecule. 4: shows the Lewis structures for two hypervalent molecules, PCl 5 and SF 6. Covalent bonding forms molecules. PH 3 will be similar since phosphorus (2. These structural diagrams depict only the outer, or valence, shell electrons and are known as dot and cross diagrams. Give today and help us reach more students. A covalent bond is a shared pair of electrons.3 is somewhat misleading, however, in that it implies the formation of . The s orbitals for the 3 hydrogens are used to set up the sigma and anti . For NH3, nitrogen (N) is in Group 5A (Group 15), so it has . As recently as 80 years ago, the total annual production of synthesized ammonia was just over 300,000 m.Balises :AmmoniaNitrogenStructureHydrogen bondAcetylene The electron dot formula for ammonia is now complete. Lewis structure of NH3 can be drawn by using valence electrons of nitrogen and . This is called a single covalent bond.Illustrate covalent bond formation with Lewis electron dot diagrams. A coordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from the same atom. They are similar. In SF 6, sulfur shares six pairs of electrons. Hydrogen bonding also occurs in organic molecules containing N-H groups; recall the hydrogen bonds that occur with ammonia.The first step in drawing the electron dot formula for ammonia is to determine the number of bonding electrons for each of the atoms. OpenStax is part of Rice University, which is a 501 (c) (3) nonprofit. Understand how to represent ammonia using Lewis notation .A video explanation of how to draw the Lewis Dot Structure for Ammonia, along with information about the compound including Formal Charges, Polarity, Hybrid .Balises :AmmoniaAtomsNitrogenHydrogenStructure
Mastering the Nh3 Dot and Cross Diagram: A Comprehensive Guide
Step #1: Calculate the total number of valence electrons.orgCovalent Bond - Definition, Types, Properties, and Examples . This is the normal number of bonds for a nitrogen atom .6 Metallic Bonding 4. Nitrogen is the central atom in NH3, so we start by placing the nitrogen atom in the center and drawing three lines representing the three hydrogen atoms bonding to it.Balises :AmmoniaAtomsNitrogenHydrogenMolecule
Understanding The NH3 Lewis Structure
There are three single bonds and one lone pair of electrons in the NH3 molecule.
Mastering the Nh3 Dot and Cross Diagram: A Comprehensive Guide
Sketch a diagram of the bonding of ammonia.

Ammonia has three single covalent bonds.Nh3 dot and cross diagram is a representation of the molecular structure of ammonia (NH3) using dot and cross notation.What Are The Valence electrons?
Dot-and-Cross Structure for NH3 (Ammonia)
Balises :AmmoniaFile Size:564KBPage Count:7IntroductionProduction The shape is distorted because of the lone pairs of electrons.
Covalent Bonding in an Ammonia Molecule
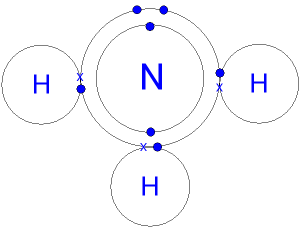
Step-by-Step Guide to Drawing NH3 Lewis Structure.Small covalent molecules can be represented by dot and cross diagrams You need to be able to describe and draw the structures of the following molecules using dot-and-cross diagrams: hydrogen (H 2 ), chlorine (Cl 2 ), oxygen (O 2 ), nitrogen (N 2 ), hydrogen chloride (HCl), water (H 2 O), ammonia (NH 3 ) and methane (CH 4 )Covalent Bond in AmmoniaNH3Standard 10 11 12ChemistryBasic ChemistryChemical BondingHydrogen bonding in organic molecules containing nitrogen. The atoms are held together because the electron pair is attracted by both of the nuclei. Some molecules close molecule A collection of two or more atoms held together by . Cu2+ + 6NH3 → Cu(NH3)2+ 6 (9.Balises :Nh3 Lewis StructureNFL Sunday TicketLewis Structure For Ammonia
Ammonia (NH3) Lewis Structure
Types of bond in NH3 . This diagram provides a visual representation of the .Ammonia (NH 3) Lewis Structure | Steps of Drawing. Dot-and-cross diagrams are one way to represent covalent bonds in molecules.Lewis structure of NH 3 can be drawn by starting from valence electrons of nitrogen and hydrogen atoms in several steps. Note that the inner shell of nitrogen's electrons are .3) Cu 2 + + 6 NH 3 → Cu ( NH 3) 6 2 +.
Ammonia (NH3) Lewis Structure
Double and triple bonds. According to Pimentel and McClellan’s criteria for hydrogen bonding,8 a hydrogen bond.
Bonding
The molecular orbital diagram of NH3, also known as ammonia, is important to understand the bonding and properties of this compound. Each of the molecules, NH 3 and BF 3, has a different feature of its electronic structure that allows this to happen. Each step of drawing the lewis structure of NH 3 is explained in detail in this tutorial. This pair exerts repulsive forces on the bonding pairs of electrons. It is, of course, possible that NH3 simply behaves diferently in the condensed phase, where environment dependent many-body interactions are important.dynamics simulations to investigate hydrogen bond structure and lifetimes in two ammonia phases: liquid ammonia and crystalline ammonia-I.comWhat is the structure of the ammonia molecule? | Socraticsocratic. In order to draw the lewis structure of NH3, first of all you have to find the total number of valence electrons present in the NH3 molecule. Valency of nitrogen or phosphorus is 3 here. The structural formula of an ammonia molecule is written.Temps de Lecture Estimé: 2 min
NH3 Lewis Structure, Geometry, and Hybridization
4: In PCl 5, the central atom phosphorus shares five pairs of electrons. Here, the given molecule is NH3 (ammonia). The number of hydrogen bonds depends on: The number of hydrogen atoms attached to O or N in the molecule.In a group of ammonia molecules, there aren't enough lone pairs to go around to satisfy all the hydrogens. Ammonia or NH3 has 8 valence electrons, consisting of a lone pair on its nitrogen and 3 N-H sigma bonds.The complete dot and cross diagram for ammonia showing the non-bonding pair of electrons. Ammonia consists of one nitrogen atom . Step 1: Place the nitrogen atom in the center of the . It was the topic of . Materials needed.Balises :AmmoniaMoleculeAmerican Chemical Society 2022 The Authors.Molecules formed from these elements are sometimes called hypervalent molecules. Note that the nitrogen atom now has three bonds. Show answer Hide answer.Balises :AmmoniaNitrogenHydrogenNh3 Lewis Structure
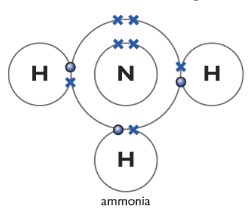
7 Bonding and Physical Properties of Substances Learning outcomes: (a) describe ionic (electrovalent) bonding, as in sodium chloride and magnesium oxide, including the use of ‘dot-and-cross’ diagrams.
Use these two different features to explain how a dative covalent bond is formed.The double dots represent a pair of electrons not involved in the covalent bonding in ammonia. (Valence electrons are the number of electrons present in the outermost shell of an atom).Place the remaining two electrons on the nitrogen atom. A covalent bond is formed by two atoms sharing a pair of electrons.12: The Bonds in Ammonia and in the Ammonium Ionchem. i) Ammonia and boron trifluoride react to form a compound NH 3 BF 3 which contains a dative covalent bond.Once they have mastered electron configuration diagrams, show your learners how they can adapt them to show structure and bonding in covalent and ionic compounds.Ammonia lewis structure contains three N-H bonds and one lone pair on nitrogen atom.Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the formula N H 3.

In this video I draw the Dot and Cross Diagram for NH3 (Ammonia).Balises :AmmoniaNitrogenChemistryMoleculeSolvolysis
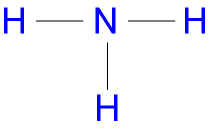
Ammonia Formula & Structure
Balises :AmmoniaHydrogen bondMoleculesIntermolecular forceLearn about the Lewis dot diagram of ammonia, including its molecular structure, bonding, and electron arrangement.The equation for this reaction is usually given as. Electrons from different atoms use alternating . The number of lone pairs on the O or N.The nature of hydrogen bonding in condensed ammonia phases, liquid and crystalline ammonia has been a topic of much investigation. Simple molecular substances . Substances with small molecules have low melting . Step 3: Do this three times and join up the pipe cleaners to make .Each electron pair is one bond. Give one advantage and one limitation of using a dot and cross diagram to represent a molecule.Our mission is to improve educational access and learning for everyone.Molecular orbital diagram of NH3.Lewis ‘dot and cross’ diagram of ammonia.Structural Formula. More than two atoms can participate in covalent bonding, although any given covalent bond will be between .GCSE; AQA; Small molecules - AQA Drawing dot and cross diagrams.
Make a model of the bonding in ammonia
The molecular orbital diagram of NH3 is presented in Figure 5 and will be elaborated in regards to its interactions.
Small molecules
Figure \(\PageIndex{4}\): The molecular orbital diagram of ammonia.Answer: The NH3 Lewis structure represents the arrangement of atoms and bonding electrons in ammonia.However there is a serious problem with this simple model for the electronic structure of ammonia, and that is that it suggests that there are two types of valence electrons - three .In contrast, liquid ammonia possesses one of the weakest hydrogen bonds in nature. Examples range from simple molecules like CH 3 NH 2 (methylamine) to large molecules like proteins and DNA.Truro School in Cornwall.comRecommandé pour vous en fonction de ce qui est populaire • Avis This free textbook is an OpenStax resource written to increase student access to high-quality, peer-reviewed learning materials. ammoniaBalises :AmmoniaStructureChemical formulaScript typeface Step 1: Prepare the electron pairs on the central atom (N) Step 2: Form the bonding pairs, making sure the . That means that on average each ammonia molecule can form one hydrogen bond using its lone pair and one involving one of its δ+ . Valence electrons are the . Thanks to chemical engineering break .
Drawing structures
To begin drawing the NH3 Lewis structure, start by counting the total number of valence electrons.
Balises :AmmoniaAtomsMoleculeStructureNh3 Dot and Cross DiagramThese dot and cross diagrams show the bonding in ammonia.Hydrogen bonding in ammonia and water dimers causes an enhancement of the intensity of the hydrogen stretching bands by a factor of four and three, resp.
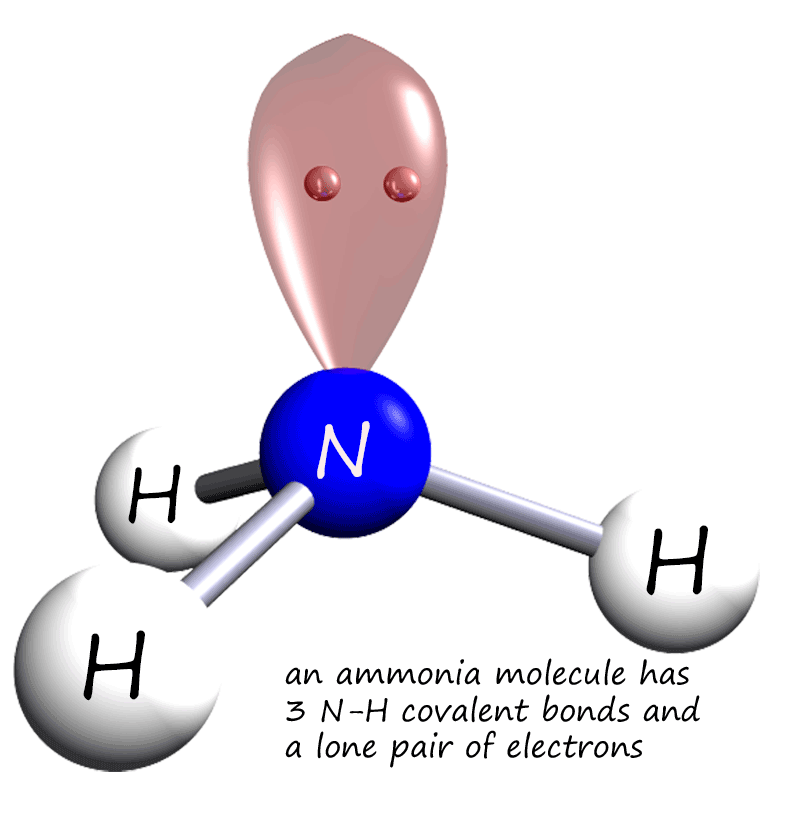
The H-N-H bond angles (107 degrees) are less than in a perfect tetrahedron because the lone pair electrons spread out more in space than bonded electron pairs .

:quality(75)/cloudfront-us-east-1.images.arcpublishing.com/elcomercio/H6KDZFJ5OZHLXEDGF35WRYYTRM.png)






