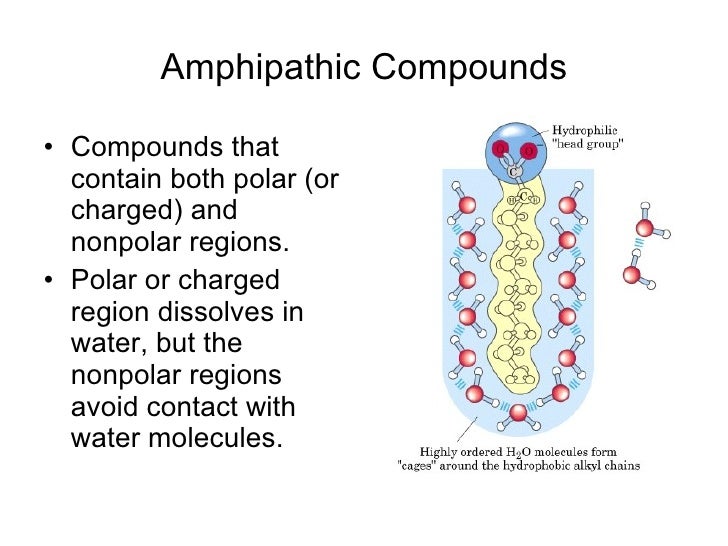
Amphipathic molecules contain components
Amphipathic molecules; Amphiphiles; Amphiphilic block copolymers; Amphiphilic polymers; Amphipols; Detergents; Emulsifiers; Hydrotropes; Lipids/phospholipids; Soaps; Surfactants. The hydrophilic head can interact with the polar molecules while the hydrophobic tail avoids polar . Notice the “R” designation within the hydrophilic head depicted in Figure \(\PageIndex{3}\), indicating that a polar head group can be more complex than a simple phosphate moiety. However, instead of using polymeric chain(s) in the cases of NDs and .The bicellar system contains the favorable characteristics of both detergent micelles and lipid bilayers.An amphipathic molecule is a molecule that has both polar and non-polar parts.Temps de Lecture Estimé: 8 min Asymmetric effects of amphipathic molecules on mechanosensitive channels. Preface; The Chemistry of . Amphipathic molecules contain both polar (having a dipole) and nonpolar parts.” The number of carbons in the fatty acid may range .Points clés à retenir : molécules amphipathiques.
Solved Insert the correct words into the sentences regarding
amphipathic molecules have both _____ regions and _____ .Membrane Lipids Are Amphipathic Molecules, Most of which Spontaneously Form Bilayers.Overview
Amphipathic
The plasma membrane is made up of two layers of phospholipids.In this tutorial/lecture we will look at the definition of amphipathic .Ethanol is an amphipathic molecule; it has both polar and nonpolar ends.Critiques : 1
Definition, Examples
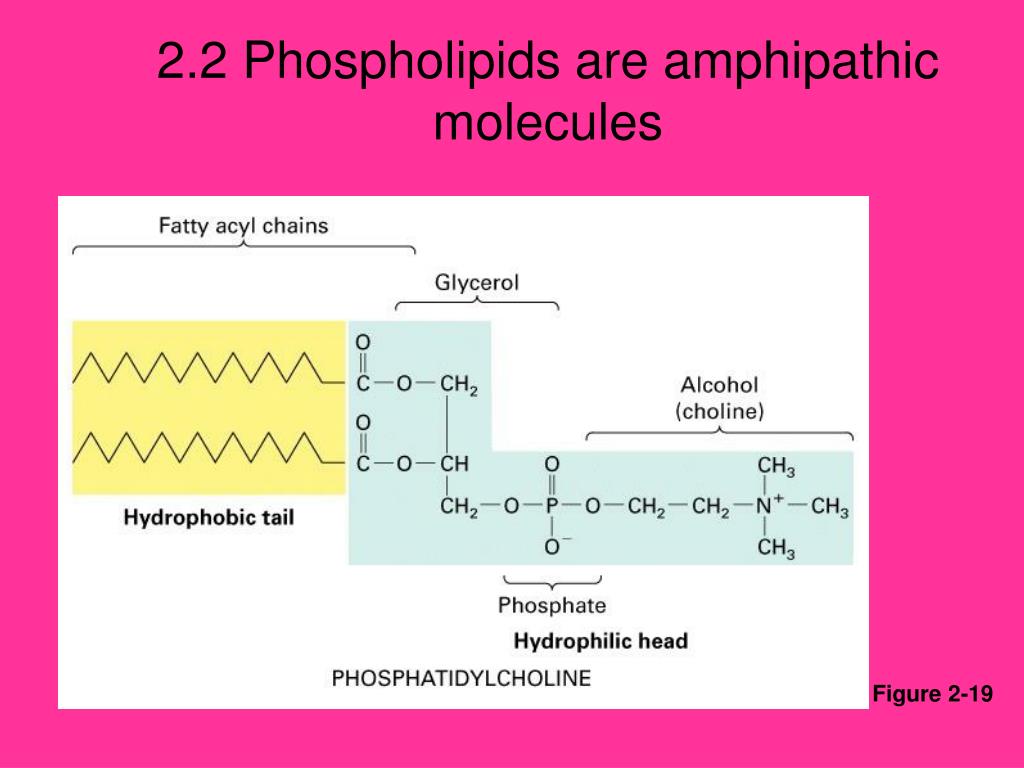
Notice the “R” designation within the hydrophilic head depicted in Figure \(\PageIndex{2}\), indicating that a polar head group can be more complex than a simple phosphate moiety. Phospholipids, for example, have non-polar fatty .

Amphiphilicity is a feature of polar contrast between the groups that make up a molecule and their spatial separation. Glycerol is an organic compound (alcohol) with three carbons, five hydrogens, a. The membrane lipids are amphipathic: part of the molecule is hydrophilic, and part of the molecule is . The other end, which consists of the fatty acids, is neutral; it is hydrophobic and water-insoluble but is fat-soluble. For phospholipids, the phosphate region carries a negative charge, making it polar, while the lipid tail is a nonpolar hydrocarbon.-Amphipathic molecules contain polar and nonpolar components.Each phospholipid molecule is an amphipathic molecule (polar at one end and non-polar at the other end).The chief components of the cell or plasma membrane are phospholipids. One type of lipid, the triglycerides, is sequestered as fat in adipose cells, which serve as the energy-storage depot for organisms and also .Amphiphilic molecules such as surfactants, lipids, copolymers, and proteins can associate into a variety of structures in aqueous solutions that can transform from one to another . Glycerol is an organic compound (an alcohol) that contains three carbons, five hydrogens, and three hydroxyl (OH) groups. The hydrophilic portion can dissolve in water while the hydrophobic portion can trap grease in micelles that then can be washed away.The bilayer cell membrane is also termed as “ Plasma membrane ”. Contents Contents. The most important classes of amphiphiles . a solution is composed of ____ dissolved in a _____ solutes, solvent.Lipids are important components of biological membranes.A phospholipid is an amphipathic molecule, meaning it has a hydrophobic part and a hydrophilic part. Figure \(\PageIndex{1}\): .Auteur : Joao's Lab Image modified from OpenStax Biology. Glycerol is an organic compound (alcohol) with three carbons, five hydrogens, and three hydroxyl (OH) groups. A cell membrane primarily composed of 40% lipid, 60% protein and 1-10% of carbohydrate of dry cell weight. interactive 3D image of a membrane phospholipid (BioTopics) Notice .
Amphipathic
Describing a molecule that has two groups with characteristically different properties, such as a detergent that has both a water-soluble (hydrophilic) polar . A similar process occurs .
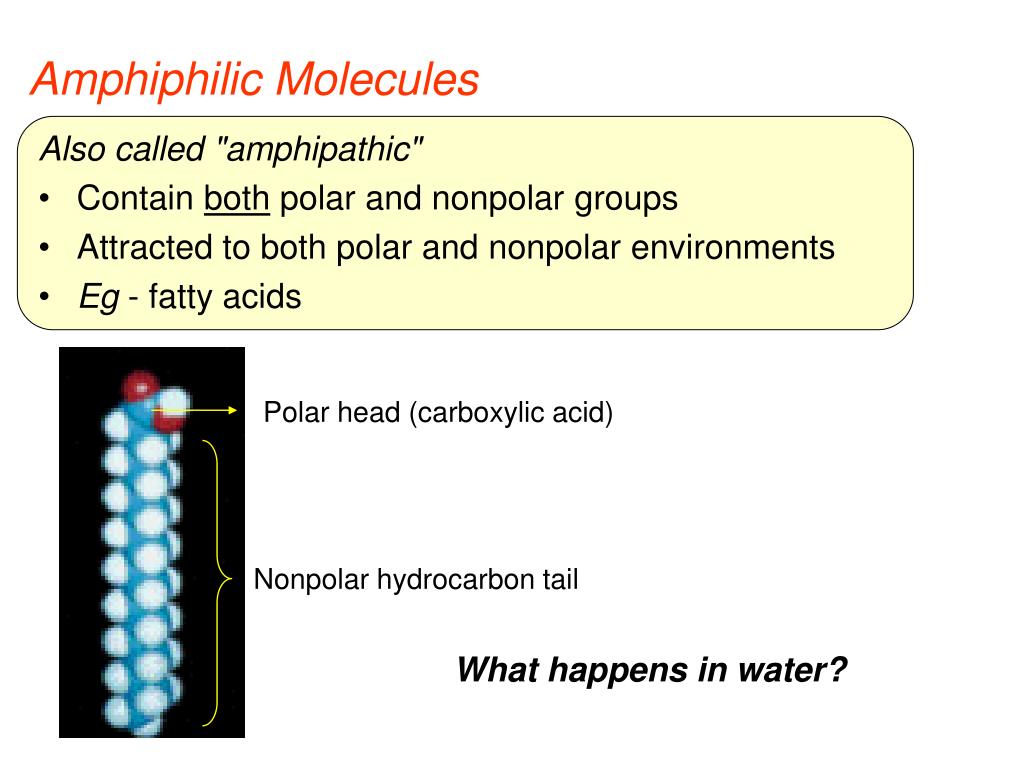
The most important property of molecules is ‘polarity’ . Ionic character of the polar head group forms the basis for the broad classification of detergents; they may be ionic (charged, either anionic or cationic), non-ionic (uncharged), or zwitterionic .Study with Quizlet and memorize flashcards containing terms like Hydrophilic and Hydrophobic Substances, Principle of Amphipathic Molecules, Major Components of . Amphiphilic molecules is a general term that describes any compound that contains two distinct covalently bonded components with .” The number of carbons in the .Therefore, phospholipids form an excellent two-layer cell membrane that separates fluid within the cell from the fluid outside of the cell. Some phospholipids, when degraded by an enzyme, produce products that act as second messengers in signal transduction. describe hydrophilic molecules .
what type of bonds are most likely to be found in a hydrophobic molecule.
Introduction To Amphipathic Molecules-Functions-Examples
A phospholipid is a lipid made of glycerol, two fatty acid tails, and a phosphate-linked head group.-An example of an amphipathic molecule is a phospholipid molecule. Les molécules amphipathiques ou amphiphiles ont des parties polaires et non polaires, ce qui les rend à .Published: 15 June 2022.

Mehdi Vaez Allaei, .A fat molecule consists of two main components—glycerol and fatty acids.
High School Biology
Image modified from OpenStax Biology.
The Lipid Bilayer
Structure and Properties
Que sont les molécules amphipathiques
It is made of a phospholipid bilayer, along with other various lipids, proteins, and carbohydrates. Table of contents., cell membranes, and these function to act as barriers to regulate the entry and exit of molecules to and from the cell. it serves as a selectively permeable membrane. Describe phospholipids .6: Non-Membrane Lipid Assemblies (Micelles) is shared under a license and was authored, remixed, and/or curated by LibreTexts.Phospholipids are amphipathic molecules that make up the bilayer of the plasma membrane and keep the membrane fluid.Amphipathic molecules have both polar and nonpolar sections.A molecule presenting a hydrophobic portion and a hydrophilic moiety is said to be amphipathic.An amphipathic molecule is a compound comprising a hydrophilic head and a hydrophobic tail. Not all terms will be used.() This molecule and related iTADs (2, 3) were designed to emulate amphipathic TADs, with hydrophobic and polar functional groups displayed on a conformationally constrained scaffold similar . This explains why plasma membranes form.Phospholipids make up the structural component of most biological membranes, e. Fatty acids have a long chain of hydrocarbons to which a carboxyl group is attached, hence the name “fatty acid.The lipid (fat) molecules that make up membranes are amphipathic: they have a charged, hydrophilic ‘head’ and a hydrophobic hydrocarbon ‘tail’.

, has an electric charge, and is attracted to water (hydrophilic). Amphipathic molecules contain polar and nonpolar components phospholipid only polar An example of an amphipathic molecule is a glucose molecule polar and onpolar The phospholipid region of an amphipathic .
Amphipathic Molecules: Components of Blood Flashcards
In fact, soap works to remove oil and grease stains because it has amphipathic properties.Detergents are amphipathic molecules, meaning they contain both a non-polar tail having an aliphatic or aromatic character and a polar head. The ethanol emulsion test works because of the amphipathic nature of ethanol. Omid Bavi, Zijing Zhou, Navid Bavi, S.The polar region of an amphipathic . nonpolar covalent. Phospholipids interact with various molecules depending upon polarity.Define Amphipathic: An amphipathic is defined as a molecule which has both polar and non-polar parts. The fatty acid chains are hydrophobic and do not interact with water, . The latter dependence is characterized by the standard enthalpy of micelle formation, Δmic H0 Δ mic H 0, where, Δmic G0 = Δmic H0 −TΔmic S0 (1. The polar ends are able to face the aqueous environment .An amphipathic molecule is one that contains both a hydrophilic and a hydrophobic region. When lipids are present in a sample . The principal components of the plasma membrane are lipids (phospholipids and cholesterol), proteins, and carbohydrate groups that are attached to some of the lipids and proteins.The principal components of the plasma membrane are lipids (phospholipids and cholesterol), proteins, and carbohydrate groups that are attached to some of the lipids .Phospholipids are major components of the plasma membrane, the outermost layer of animal cells.
Manquant :
This amphipathic nature (containing both hydrophobic and hydrophilic .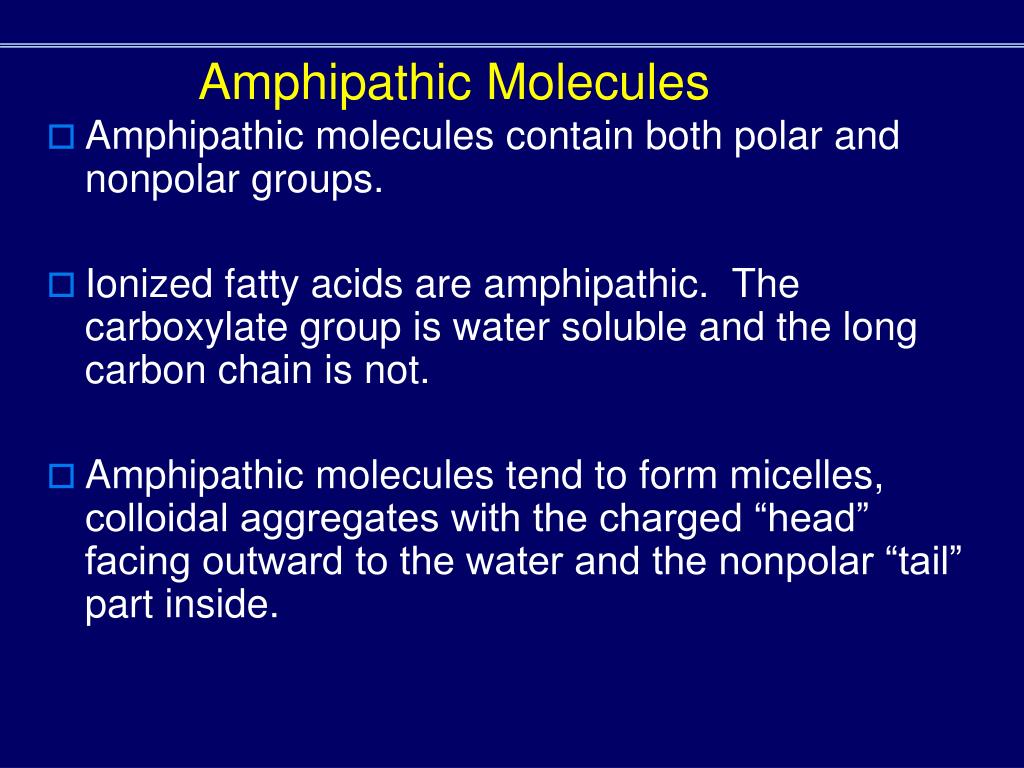
The hydrophilic head group consists of a phosphate-containing group attached to a glycerol molecule.which component of humans has the greatest percent of water. The hydrophilic portion can dissolve in the wash water while the hydrophobic portion can trap grease in stains that then can be washed away.Regarder la vidéo3:43An amphipathic molecule is a molecule that has both hydrophobic and hydrophilic parts. interactive 3D image of a .We recently reported the first small molecule that reconstitutes the function of a transcriptional activation domain, isoxazolidine TAD (iTAD) 1 (Figure 1b).Question: Insert the correct words into the sentences regarding amphipathic molecules.Amphipathic compounds (detergents) are a unique set of molecules with the ability of manipulation (distortion or formation) of the hydrophobic-hydrophilic interactions in . Like fats, they are composed of fatty acid chains attached to a glycerol . Bicelles have structural similarities to NDs and lipodisq particles because of the presence of a lipid patch wrapped by amphipathic molecules at the rim of the discs. Lipid—that is, fatty—molecules constitute about 50% of the mass of most .

Glycolipids are examples in which carbohydrates are bonded to the . Specialized structure that surrounds the cell and its internal environment; controls movement of substances into/out of cell.
Manquant :
componentsAmphiphile
Amphiphilic Molecule
BIOL 2401 Flashcards
For example, phosphatidylcholine, a common component of membranes, contains two fatty acids . Clearly the definition of . Skip to Content Go to accessibility page Keyboard shortcuts menu.On one end of the molecule are the phosphate group and one alcohol; this end is polar, i. Because of the nonpolar component of the molecule, ethanol can dissolve lipids; however, because of it’s polar component, ethanol can also mix with water.