Amplatzer amulet vs watchman

2021;144:1543–1552.

In that context, to prevent stroke in patients with non-valvular atrial fibrillation, the Amplatzer AMULET device was non-inferior in both safety and efficacy compared with the WATCHMAN. Objective: To evaluate . The Newcastle-Ottawa Scale was used to appraise study quality.5 has been replaced by the new-generation .Amulet LAAC device was associated with increased periprocedural complications as compared to Watchman LAAC device.
Results: Six studies encompassing 614 .Conclusions: The Amulet occluder was noninferior for safety and effectiveness of stroke prevention for nonvalvular atrial fibrillation compared with the .Auteur : Roberto Galea, Federico De Marco, Nicolas Meneveau, Adel Aminian, Frédéric Anselme, Christoph Gräni,. The absence of an additional disk in the .5 and Amplatzer Amulet are the two most popular used devices for preventing stroke in patients with NVAF.There are currently two FDA-approved endocardial closure devices, namely, the Watchman FLX and Amplatzer Amulet.The Amulet occluder was noninferior for safety and effectiveness of stroke prevention for nonvalvular atrial fibrillation compared with the Watchman device and .Introduction: The Amplatzer and Watchman left atrial appendage closure (LAAC) devices are the two most frequently used devices for LAAC devices worldwide.057063 Link Google Scholar; 35.002) were significantly higher among patients with Amplatzer Amulet compared to . 2022 Jun;19 (6):1017-1018. Keywords: Left atrial appendage . The Watchman and the Amulet are the two most frequently used devices for LAAC but no randomized study has so far assessed their comparative leak rates after intervention.1007/s12265-020-10095-4 Crossref Medline Google Scholar; 10.5) TM presented several limitations, such as challenges in implantation within complex left atrial appendage (LAA) anatomies, higher incidence of peri-device leak, device recapture, and device-related thrombus (DRT).4% with the Watchman device, leading to .Watchman FLX showed significantly better complete LAA occlusion at eight weeks compared to the Amplatzer Amulet (72.Procedural and short-term follow-up outcomes of Amplatzer Amulet occluder versus Watchman FLX device: A meta-analysis. Clinical Topics: Anticoagulation Management, .COMPARATIVE EFFICACY AND SAFETY OF AMPLATZER AMULET VS WATCHMAN FOR LEFT ATRIAL APPENDAGE OCCLUSION: A SYSTEMATIC REVIEW AND META .
Manquant :
watchman Current evidence suggests that percutaneous occlusion of the Left Atrial Appendage (LAA) reduces the risk of thromboembolic complications associated with non-valvular atrial fibrillation. Two consecutive cohorts were enrolled, one treated with the Amplatzer .Design and rationale of the SWISS-APERO randomized clinical Trial: comparison of Amplatzer Amulet vs Watchman device in patients undergoing left atrial appendage closure. Seven patients developed post-procedural iatrogenic atrial septal defects (four in the Watchman group vs three in the Amulet group, p-value=0.The Amulet IDE trial (Amplatzer Amulet Left Atrial Appendage Occluder IDE Trial) is the first large-scale randomized, . DJ Lakkireddy at the European Society of Cardiology Virtual Congress, August 30, 2021. The SWISS-APERO trial showed that Amulet is not superior to Watchman for LAA patency . We sought to compare safety . Presented by Dr.Percutaneous Transcatheter Occlusion.
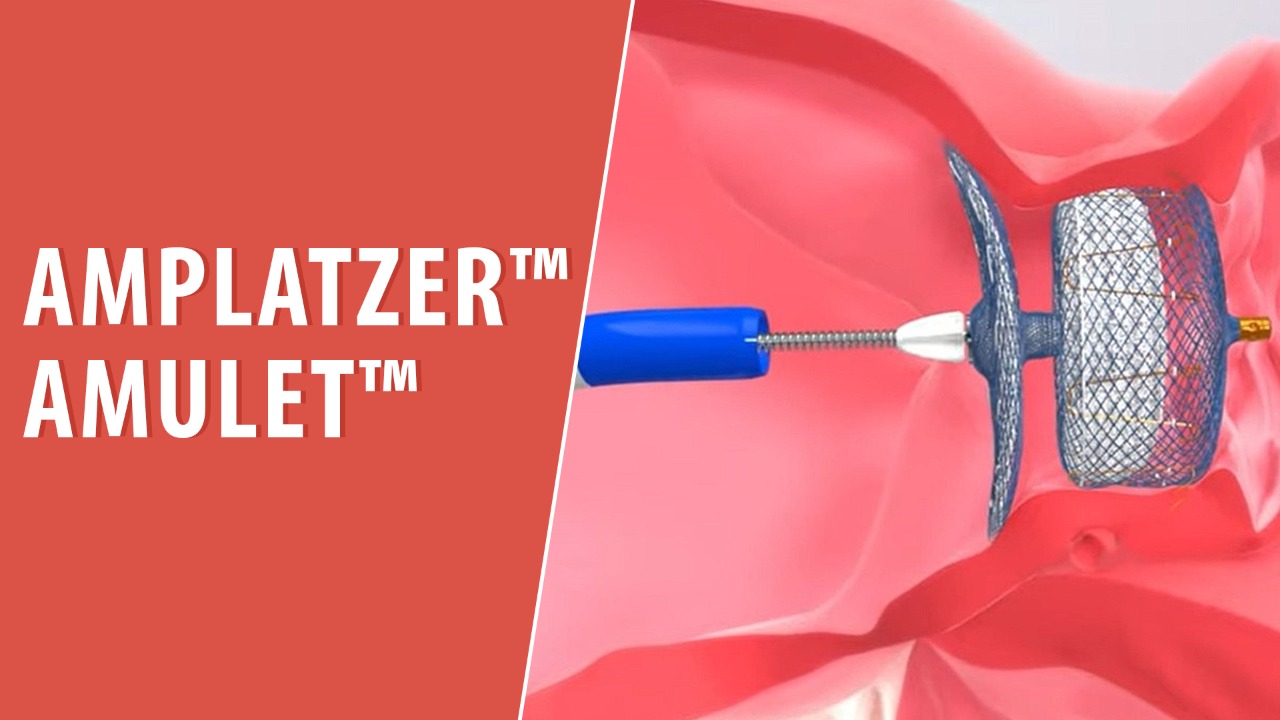
the Watchman TM device (21–26). 1 The Amplatzer™ Amulet™ LAA Occluder is designed for complete occlusion of the LAA, thereby reducing the risk . Methods Single-center cohort study of patients undergoing LAAO between 2017 and 2020.Objectives: To compare the efficacy and safety of the Amplatzer and Watchman TM LAA closure devices.Recently receiving Food and Drug Administration approval, the Amplatzer Amulet™ left atrial appendage occluder device (Abbott Medical Inc. This step-by-step .Design and rationale of the Swiss-Apero randomized clinical trial: comparison of Amplatzer Amulet vs Watchman device in patients undergoing left atrial appendage closure.In contrast, procedure-related complications (OR, 2.5 device for embolic stroke prevention in patients with nonvalvular atrial fibrillation. Amplatzer™ Amulet™ Left Atrial Appendage Occluder versus Watchman™ device for stroke prophylaxis (Amulet IDE): A randomized controlled trial. Amplatzer devices for left atrial appendage closure (LAAC).Of patients who had moderate leaks at 45 days, those treated with the Amulet versus the Watchman 2., medical director, structural heart program, Banner University Medical Heart Institute, Phoenix, explains the difference he found between the FDA cleared Watchman left atrial appendage (LAA) occluder and the Abbott Amplatzer Amulet device now in U.We assessed the safety and efficacy of LAAO using the Amplatzer Amulet and Watchman. He spoke on these differences at the 2018 .Ashish Pershad, M.A total of 1,878 patients were randomized from September 2016 through March 2019, and 1,833 underwent a device implant attempt (917 Amulet occluder group patients and 916 Watchman device group patients) (Figure 1).6%), and 3 years (3.0% follow-up rate, and patients in the Watchman .4% of the one-time procedures compared to 96. J Cardiovasc Transl Res. Recent Findings. Methods: Patients with NVAF at increased risk of stroke were randomly assigned (1:1) to undergo percutaneous .
Amplatzer Amulet Left Atrial Appendage Occluder IDE
The Amulet IDE trial (Amplatzer Amulet Left Atrial Appendage Occluder IDE Trial) was designed to evaluate the safety and effectiveness of the dual-seal mechanism of the Amulet LAA occluder compared with the Watchman device.Atrial appendage occlusion in itself improved with AMULET, but with a higher rate of procedure-related complications that decreased with .7%), 18 months (3.The dual-seal mechanism Amulet Left Atrial Appendage (LAA) Occluder continued to demonstrate safety and effectiveness in patients with nonvalvular atrial .Highlighted text has been updated as of October 24, 2023.Several cohort studies have directly compared the safety and efficacy of the Amplatzer cardiac plug or amulet vs.Amplatzer amulet left atrial appendage occluder versus watchman device for stroke prophylaxis (amulet IDE): a randomized, controlled trial.

The EWOLUTION trial for Watchman, as well as multicenter registries for Amplatzer Cardiac Plug and Amulet , reported complication rates of 2.5 were less likely to still have leaks of that size at 1 year (39% vs 46%).1007/s12265-020-10095-4 Crossref Medline Google Scholar; 4.The largest comparison of peri-procedural success and short-term outcomes of WATCHMAN FLX vs.

While this is theoretically a .Amplatzer ™ Amulet ™ Left Atrial Appendage Occluder Versus Watchman ™ Device for Stroke Prophylaxis (Amulet IDE): A Randomized Controlled Trial. Current data highlights .Auteur : Mohammed Saad, Osama Risha, Makoto Sano, Thomas Fink, Christian-Hendrik Heeger, Julia Vogler, Vaness.At 3 years, patients in the Amulet occluder group had a 92. Ellis, Vijendra Swarup, Lars Sondergaard, John C.
Comparison between Amplatzer and Watchman Left Atrial
However, this comparison does not reflect current clinical practice, as the old Watchman 2.

(AMPLATZER Amulet LAA Occluder Trial .Aims: This study compares clinical outcomes of Watchman vs.Watchman FLX achieved a near 100% implantation success and a substantial decrease in the occurrence of periprocedural complications as compared to .Results: The study included 37 patients (21 had Watchman devices, 16 had Amplatzer Amulet LAA Occluder devices, and 28 were men, mean age 76.Residual or newly acquired leaks are routinely appraised after left atrial appendage closure (LAAC). 2021; 144:1543–1552. Three patients .Latest data continue to show the benefits of Amplatzer™ Amulet™ LAA Occluder's immediate and complete closure of the LAA compared to Watchman‡ Findings underscore the importance of innovative, minimally invasive treatment options for people battling complex heart conditions like aortic stenosis and atrial fibrillation; ABBOTT .5 and Amplatzer Amulet in patients undergoing LAAC procedure.Auteur : Dhanunjaya Lakkireddy, David Thaler, Christopher R.
Design and Rationale of the Swiss-Apero Randomized Clinical
which showed superiority for LAAO (lower rates of leaks >5 mm) based on a comparison between the second-generation Amplatzer TM plug, namely, the Amulet TM occluder, and the old-generation Watchman TM 2. However, these studies were limited in sample size and therefore we conducted a meta-analysis of published data that directly compared the Amplatzer and Watchman TM devices, . Left atrial appendage closure: outcomes and . The newer generation Watchman FLX (W-FLX) TM was introduced . The “Comparison of Amplatzer Amulet vs Watchman devices . Recent SWISS APERO trial demonstrated . We sought to compare safety and efficacy outcomes between Watchman 2. Amplatzer Amulet reveals superior procedural safety, higher . Of two real-world .The recent Amulet IDE trial (AMPLATZER Amulet LAA Occluder Trial) was the first head-to-head randomized comparison of .The Amplatzer Amulet (AA) and Watchman devices (WD) are the 2 most frequently used devices for percutaneous LAA closure globally. The Newcastle-Ottawa Scale has been utilized to assess the quality of study.Introduction: The first-generation Watchman 2. That could be related to a higher incidence of late device-related thrombus (DRT) in the .Watchman Generation 2. van Rein N, Heide-Jørgensen U, Lijfering WM, Dekkers OM, Sørensen HT, Cannegieter SC.Learn about Abbott's Amplatzer Amulet Left Atrial Appendage Occluder, a minimally invasive device to prevent blood clots and minimize stroke risk in people with atrial .Background The left atrial appendage (LAA) sealing properties of the Amplatzer Amulet and Watchman FLX devices were compared using cardiac computed tomography (CT) follow-up.The Amplatzer™ Amulet™ LAA Occluder IDE Trial (Amulet IDE Trial) was designed to evaluate the safety and effectiveness of the dual-seal mechanism of the Amulet LAA occluder compared with the Watchman™ device. Amplatzer Amulet LAA Occluder Instructions for Use. “The dual-seal Amplatzer Amulet left atrial appendage closure device demonstrated durably superior closure at 12 months compared to the single-occlusive mechanism .Device based LAA closure rates were higher and demonstrated superiority of the Amulet LAA occluder compared with the Watchman device (98.

The Watchman and Amplatzer Amulet devices remain vital for left-atrial appendage closure (LAAC). Methods and results. Lakkireddy D, et al. METHODS: Patients with nonvalvular atrial fibrillation at increased risk of stroke were randomly assigned (1:1) to undergo .) provides a dual seal mechanism alternative to the commercially available Watchman FLX or Watchman 2.One-Year Outcomes After Amulet or Watchman Device for Percutaneous Left Atrial Appendage Closure: A Prespecified Analysis of the SWISS-APERO .2%, respectively.This study compares clinical outcomes of Watchman vs.Use of oral anticoagulation was lower in patients treated with the Amplatzer Amulet versus the Watchman throughout follow-up, with significant differences at 6 months (2. Methods We carried out a comprehensive and systematic search of . However, these trials were not controlled, and the definitions of procedure-related complications were different.Among more than 1,800 patients with an average age of 75, the Amplatzer Amulet was correctly placed in 98. Methods: A meta-analysis was performed of studies comparing the safety and efficacy outcomes of the two devices.










