Anions chemistry pdf
Some anions like nitrate and cyanide represent common radicals. Identify each of the ions shown below.Anion chemistry in energy storage devices.Your goal in this lab is to perform a series of qualitative chemical tests – based on chemical reactivity and solubility – to determine the identity of anions in an aqueous solution of unknown composition.” Examples of anions include: Hydroxide anion: OH –.AQA Chemistry A-level Required Practical 4 Carry out simple test-tube reactions to identify cations and anions www. What are valence electrons?
Cations and Anions
Published in Chemical Society Reviews 17 September 2010. Look at Figure 9.As a long-standing Head of Science, Stewart brings a wealth of experience to creating Topic Questions and revision materials for Save My Exams.This themed collection of articles brings together aspects of modern supramolecular chemistry related to anionic species.
The supramolecular chemistry of anions
Nature Reviews Chemistry 7 , 616–631 ( 2023) Cite .1 pm , respectively.ANIONS et CATIONS I- Anion et Cation Un anion est un atome (ou une molécule) qui a gagné un ou plusieurs électrons.
Qualitative Analysis of Anions
Place 10 drops of 0.
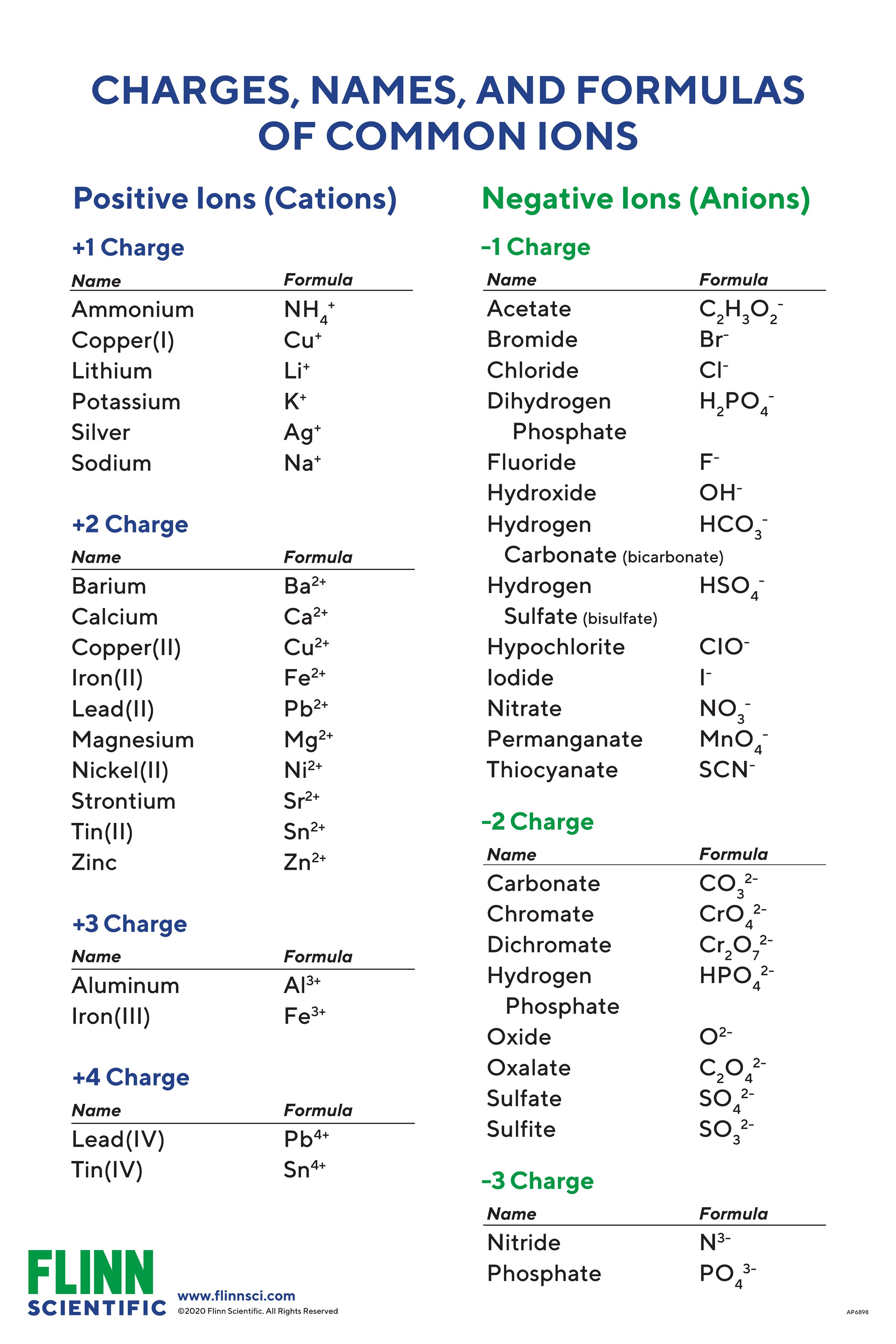
Ionic Charges Chart (Cations and Anions) Cations
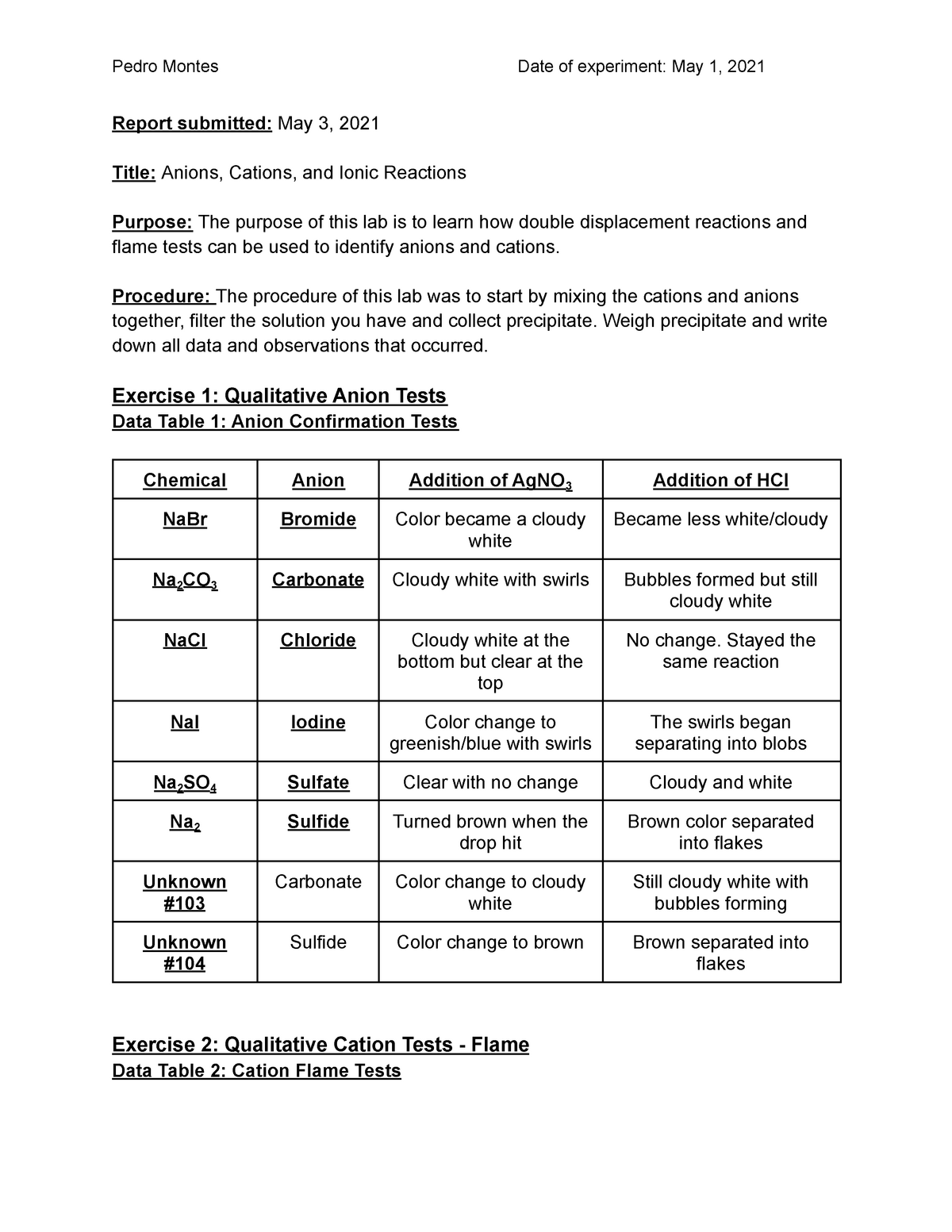
It’s common to separate out these tests into cation tests (group 1, group 2 and the ammonium ion), anion tests . This field has grown from the pioneering work of Shriver and Biallas 1 and Simmons and Park 2 in the late 1960s on Lewis acid-based anion hosts and ammonium-based cage compounds, respectively, through the 1970s and early .txt) or view presentation slides online.pdf), Text File (. If an atom loses 2 electrons it becomes a(n) _____.When electrons are transferred and ions form, ionic bonds result. | Find, read and cite all the . Beryllium ion . The tests involve adding reagents like silver nitrate, . Analysis Of Cations And Anions I. This is cobalt.Anions are negative ions that are formed when a nonmetal atom gains one or more electrons.This species has a 2− charge on it, so it is an anion. The test tube should be swirled carefully to ensure that it is mixed well.

The general formula for an acid is H n X, where X is an anion, H is a hydrogen ion, and n is the number of hydrogen ions needed to make the acid neutral.Anions 1-acetate C 2 H 3 O 2-cyanide CN-amide NH 2-cyanate OCN-hydrogen carbonate fluoride F-(bicarbonate) HCO 3-hydride H-hydrogen sulfate hydroxide OH-(bisulfate) . Now a neutral molecular cage capable of donating 12 . Amount of substance employed in these is . In order to determine the anions in the unknown crystals (any ionic compound must contain both cations and anions) a somewhat different approach that .comRecommandé pour vous en fonction de ce qui est populaire • Avis Experiment Title II. Your unknown solution will contain 2, 3, or 4 of the following anions: chloride, carbonate, iodide and sulfate. Revision notes on 9. Experiment Purpose : 1. Valence Electrons 1. Kristin Bowman-James, Prof. Formally, a carbanion is the conjugate base of a carbon acid.

Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between objects of opposite electrical charge (in this case, cations and anions). Both cations and anions are organized by their valence state with monovalent, divalent, trivalent, tetravalent, pentavalent and hexavalent . This article marks the 20th year of coverage of anion receptor chemistry by this series of reviews.formation of a salt, the part contributed by the acid is called anion and the part contributed by the base is called cation. Article Literature Review. The review discusses anion receptors that employ hydrogen bond donors (both NH and CH .TEST FOR ANIONS (CHEMISTRY) - Free download as Powerpoint Presentation (.Request PDF | Anion chemistry in energy storage devices | Anions serve as an essential component of electrolytes, whose effects have long been ignored. To simplify the scheme, we separate the cations into five .Direct experimental evidence is provided that already single unoptimized anion-π interactions can stabilize enolates by almost two pKa units, and these findings are significant because enolate chemistry is central in chemistry and biology, and they will stimulate the use of anion -π interactions in catalysis in the broadest sense. When electrons are “shared” and molecules form, covalent bonds result. Anions are so named because they are attracted to the anode (positive field) . To determine the cation which can be found in the analite 2. Record observations for each test tube and comment . Hydrogen ion (proton) Ammonium ion. Because this species has no charge, it is an atom in its elemental form. Experiment Date Group IV : Analysis of Cations and Anions : October 18th, 2014 at 07. However, for the cations in particular, there is a complex range of cations that can be tested. Write the symbol for the calcium ion. However, since the 2010s, we have seen a . Antonio Bianchi, Prof.Fluorescence and NMR titration studies exhibit that 1 has a higher affinity for anions with larger size, tetrahedral geometry, and higher electron density, resulting in an .Anions: Add additional electrons fill valence shell of the symbol. Zhaodong Huang1,2,5, Xinliang Li 2,3,5, Ze Chen2, Pei Li2, Xiulei Ji 4 & Chunyi Zhi 1,2.Regarder la vidéoThe different chemical properties and reactions of various cations and anions enable you to distinguish between them using simple laboratory chemicals. Qualitative analysis is carried out on various scales.Reading Anion Names and Formulas
Tableau récapitulatif des principaux ions de la chimie
reaction for detection of anions-oxidants, anions-reductions, anions of volatile acids.
Carbanions II
IGCSE CHEMISTRY/0620/NOTES FOR USE IN QUALITATIVE ANALYSIS 2 ©EDUCATALYST2020 Notes for use in qualitative analysis Tests for anions *precipitate = ppt. In the first review, published in Coordination Chemistry Reviews in 2000 (and covering highlights in anion coordination and anion directed assembly in 1997 and 1998) [1], it was noted that “Anion coordination has received little .Revision notes on 12. Determine the causes and learn the systematic course of analysis for .Tableau récapitulatif des principaux ions de la chimie.
NIT-7 SYSTEMATIC QUALITATIVE ANALYSIS
Temps de Lecture Estimé: 3 min
Common Anions List and Formulas
After mixing, add 2 or 3 drops of AgNO 3 ( aq) solution to both test tubes and mix well.
Common Ionic States of the Elements
Lithium ion Sodium ion Potassium ion Rubidium ion Cesium ion. Stewart specialises in Chemistry, but has also taught Physics and Environmental Systems and Societies.
Anion receptor chemistry . About 10 drops of sodium hydroxide should be added using a pipette. Aspects of Anion Coordination from Historical Perspectives (Pages: 1-73) Prof. End of The Experiment : October 18th, 2014 at 10. Chemistry, Materials Science. The cations and anions are tested separately as they dissociate when dissolved.involves the identification of the cation and anion of an inorganic salt.
ANIONS et CATIONS
4 Tests for Anions for the Edexcel GCSE Chemistry syllabus, written by the . Nature Reviews .consisting of an anion and enough hydrogen ions to make the acid electrically neutral. A connaître par cœur : . For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons.What three hydrogen-containing polyatomic anions are essential components of living systems? a.Anions are ions with a negative charge. Comme l'électron est chargé négativement, l'anion . This critical review covers advances in anion complexation in the year 2008 and 2009. Check for updates.
Salt analysis is an integral part of the CBSE class 12 chemistry practical examinations and is a topic that several students . About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a clean test tube. [1] The carbanion exists in a trigonal pyramidal geometry.
[PDF] Enolate chemistry with anion
Atoms, Molecules, and Ions Law of Constant Composition Joseph Proust (1754–1826) • Also known as the law of definite . Anion chemistry in energy storage devices. Anion charge is equal to electrons added.Very few charge-neutral synthetic anion receptors can function in water, and those known typically select weakly hydrated anions such as iodide.Li+ Na+ K+ Rb+ Cs+. Zhaodong Huang, Xinliang Li, Ze Chen, Pei Li, Xiulei Ji & Chunyi Zhi.
Common Cations, Anions, Acids, and Organic Compounds

The document describes tests to detect 7 common anions: chloride, sulfate, sulfite, carbonate, hydrogen carbonate, nitrate, and phosphate.Atoms of group 17 gain one electron and form anions with a 1− charge; atoms of group 16 gain two electrons and form ions with a 2− charge, and so on.The symbol for the element calcium is Ca, which is a metallic element, and metals in the combined form yield ionic compounds. R3C−H +B− → R3C− + H−B (1) (1) R 3 .
9: Ionic Structures (Worksheet)
Ion Magnésium.

The review focuses on the applications of anion receptor chemistry including sensing, self-assembly, extraction .pptx), PDF File (.
Anion Analysis
Anion chemistry in energy storage devices | Request PDF. After reading Lesson 7.comControles Corrigés Atomes et Ions 3eme PDF - Exercices .Anions 1-acetate C 2 H 3 O 2-cyanide CN-amide NH 2-cyanate OCN-hydrogen carbonate fluoride F-(bicarbonate) HCO 3-hydride H-hydrogen sulfate hydroxide OH-(bisulfate) HSO 4-hypochlorite ClO-bisulfide HS-iodate IO 3-bisulfite HSO 3 . Add some drops of H 2 SO 4 ( aq) to both the known chloride solution and one of the unknown crystal solutions. This results in an anion with 35 protons, 36 electrons, and a 1− charge. This is the oxide anion.
Anion chemistry in energy storage devices
Exercices Corrigés Atomes et Ions 3ème PDFexercicesgratuits.

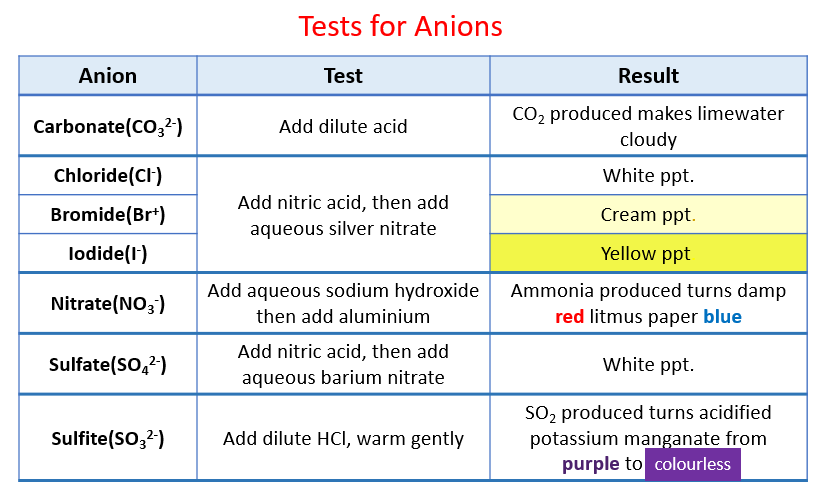
Test for halide ions in solution: Chloride (Cl–), Bromide (Br–), Iodide (I–) Test: Acidify with dilute Nitric acid, then add aqueous Silver nitrate. The test tube of the solution should then be placed in a .Examples of anions include: Hydroxide anion: OH – Oxide anion: O 2-Sulfate anion: SO 4 2-Electrons are added to form anions, so they may be larger than neutral atoms if another electron shell forms. Must be clean to ensure a clear test result.Clean the wire and then repeat flame test with sodium chloride and iron (III) chloride. Anions are grouped by their valence and include common inorganic ions such as chloride, bromide, sulfate, phosphate and hydroxide. For example, in the salts CuSO 4 and NaCl, Cu 2+ and Na + ions are cations and SO 4 2– and Cl – ions are anions.


)