Ansi aami st72

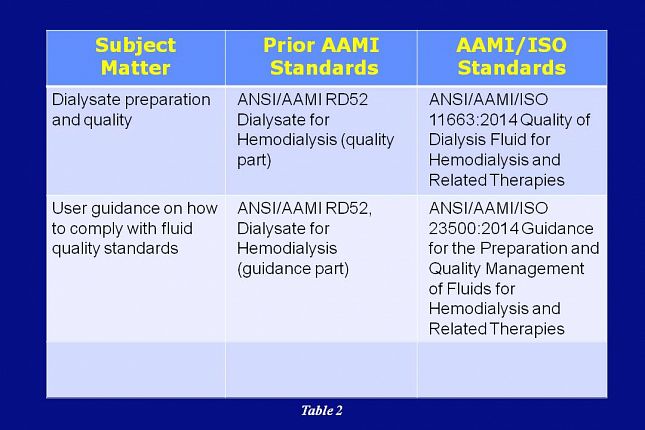
Access the ANSI/AAMI ST72 / ASTM D4169 - Bacterial Endotoxins Test Methods Set which provides general criteria for the determination of bacterial endotoxins on or in medical devices, components, or raw materials.2:2005 医用输液、输血、注射器具检验方法 第2部分:生物学试验方法 《中国药典(二部)》附录“细菌内毒素检查法 .AAMI has released four amendments to ANSI/AAMI ST79, Comprehensive guide to steam sterilization and sterility assurance in health care facilities, offering users new clarity and . Approved 11 July 2019 by . This recommended practice specifies general criteria to . Specifies general criteria to be applied in the determination of bacterial endotoxins on or in medical devices, components, or raw materials employing bacterial endotoxins test (BET) methods using amebocyte lysate reagents from Limulus . On each equation replace the denominator, “1”, with a lambda, “λ”. Also known as the “nonpyromaniacs,” the ST72 . Guidance for Industry.米国国家規格協会(ansi)/aami 基準st72 の最新改訂版は2011 であり、2002/r2010 ではないことに留意されたい。st72 の2 バー ジョンの原則は概ね一貫しているが、新しい改訂版には追加情報が 記されているため、最新バージョンの使用を推奨する。 ii. Endotoxins from gram-negative bacteria are the most common cause of toxic .ANSI/AAMI ST72:2019 Bacterial endotoxins— Test methods, routine monitoring, and alternatives to batch testing American National Standard This is a preview of . 第章2,ANSI/AAMI ST72:2019还讨 STERIS provides contract analysis of bacterial endotoxins using methods compliant with EP, USP and ANSI/AAMI ST72 to meet FDA and MHRA requirements. Sterilization of health care products - Radiation sterilization - . Additional copies are available from: Office of .ANSI/AAMI ST72 / ASTM D4169 - Bacterial Endotoxins Test Methods Set.
St ANSI AAMI ST72-2019 in English
Bacterial endotoxins — Test methods, routine monitoring, and alternatives to batch testing.

Available for Subscriptions Available in Packages.This document is based on ANSI/AAMI ST72.ANSI/AAMI ST72:2011(R2016) Bacterial endotoxins - Test methods, routine monitoring, and alternatives to batch testing.

This standard establishes .
Tests d’endotoxines bactériennes (LAL)
Specifies general criteria to be applied in .
ANSI/AAMI ST72:2011 (R2016)
ANSI/AAMI ST72:2004 (R2010) Bacterial endotoxins - Test methodologies, routine monitoring, and alternatives to batch testing.
© Copyright AAMI 201 What to Expect in the Third Edition of ST72
ANSI/AAMI ST72:2002 (R2010) Bacterial endotoxins - Test methodologies, routine monitoring, and alternatives to batch testing. Alert me in case of modifications on this product. Why is bacterial endotoxin testing important? A 2023 paper highlighted that .Sterilization of health care products - Requirements and guidance for selecting a sterility assurance level (SAL) for products labeled “sterile”. ANSI/AAMI/ISO 10993-5:2009 (R2014), Biological evaluation of medical devices—Part 5: Tests for in vitro cytotoxicity. Available for Subscriptions.ANSI/AAMI ST72:2019 Bacterial endotoxins - Test methods, routine monitoring, and alternatives to batch testing.
American National Standards
This committee is working on the revision of AAMI ST72, Bacterial endotoxins—Test methods, routine monitoring, and alternatives to batch testing. Page 7, Equation 2 and Equation 3 .STERIS propose des services contractuels d’analyse des endotoxines bactériennes en utilisant des méthodes conformes à EP, USP et ANSI/AAMI ST72 pour répondre aux .STERIS propose des services contractuels d’analyse des endotoxines bactériennes en utilisant des méthodes conformes à EP, USP et ANSI/AAMI ST72 pour répondre aux exigences de la FDA et de la MHRA. Pyrogen and Endotoxins Testing: Questions and Answers.
ANSI/AAMI ST72:2004 (R2010)
Taille du fichier : 382KB
ISO publishes standard on testing bacterial endotoxins
EXPORT CITATION.
ISO 11737-2:2019
Some sections have been restructured and extended or changed from ANSI/AAMI ST72, according to ISO.AAMI ST/WG 8, Microbiological Method – seeking users. For a complete copy of this AAMI document, contact . Association for the Advancement of Medical Instrumentation.When the revision process for ANSI/AAMI ST72:20111 began in April 2016, the goal of the Microbiological Methods Working Group (WG08) and its volunteer ST72 Endotoxin Task Group was to update the standard to reflect current industry and regulatory expectations for the testing of medical devices. American National Standard ANSI/AAMI ST72:2019 . Add to cart AAMI TIR35:2016. Association for the . 细菌内毒素规则标 准为细菌内毒素检测(BET ,Bacterial Endotoxins Test)提供了指导,并规定了使用鲎试剂细菌内毒素 检测法来检测医疗器械、部件、原材料上的细菌内毒 素含量的通用标准1。 正如. Testing is performed according to USP , USP and ANSI/AAMI ST72. Les tests sont effectués conformément à USP , USP et ANSI/AAMI ST72.ANSI/AAMI ST72:2011 Bacterial endotoxin - Test methods, routine monitoring and alternatives to batch testing.ANSI/AAMI ST77:2013 (R2018) Containment devices for reusable medical device sterilization. Select the citation format you wish to export for this article or chapter. What to Expect in the Third Edition of ST72.
试验方法、常规监测和批量试验变更(含勘误: 2020年4月16日) Bacterial endotoxins- Test methods, routine monitoring, and alternatives to batch testing (Includes Errata: April 16, 2020) 首页 ; 资源检索. Sterilization of health care products - Radiation - Substantiation of a selected sterilization dose at a specified . Proezd Molodezhniy, house 3, office 225 141501 Moscow region, Solnechnogorsk city, Russia .ANSI AAMI ST72細菌内毒素の標準試験、試験方法、定期的なモニタリング、およびバッチ試験の代替 .ANSI/AAMI ST72:2019 Bacterial endotoxins - Test methods, routine monitoring, and alternatives to batch testing; YY/T 0618-2017 医疗器械细菌内毒素试验方法 常规监控与跳批检验 ; USP European Pharmacopoeia GB 14233.(ANSI/AAMI/ISO ST72:2019) Erratum issued: 16 April 2020 . This standard is also available in these packages: ISO 11737 - Sterilization of Medical Devices Package; Amendments & Corrections.
Sterile Device Endotoxin Testing
ANSI/AAMI ST72:2019, Bacterial endotoxin—Test methods, routine monitoring, and alternatives to batch testing.ANSI AAMI ST72:2019 Bacterial endotoxins - Test methods, routine monitoring, and alternatives to batch testing Parker Authors Info & Affiliations.
ANSI/AAMI ST72 / ASTM D4169
Bacterial endotoxins - Test methods, routine monitoring, and alternatives to batch testing .
ANSI AAMI ST79 2017
Bacterial endotoxins - Test methods, routine monitoring, and alternatives to batch testing. Bacterial endotoxins - Test methodologies, routine monitoring, and alternatives to batch testing.
.jpg)
AAMI/CN/WG01, Luer activated valves. Availability: In stock.Buy St ANSI AAMI ST72-2019 | Delivery English version: 1 business day | Price: 35 USD | Document status: Active | ️ Translations ️ Originals ️ Low prices ️ PDF by email +44 7727 457207 (Telegram, WhatsApp) info@meganorms. contact us; Name Support Language Availability Edition date Price; ANSI/AAMI ST72:2019 : PDF : English : Active : 1/1/2019 : .This is a preview edition of an AAMI guidance document and is of the document before making a purchasing decision.ANSI/AAMI ST72:2019; Bacterial endotoxins—Test methods, routine monitoring, and alternatives to batch testing. ANSI/AAMI ST72:2002.《ANSI/ST72-2019》 细菌内毒素.BSR/AAMI ST72-201x, Bacterial endotoxins - Test methods, routine monitoring, and alternatives to batch testing (revision of ANSI/AAMI ST72-2011 (R2016)) Specifies general criteria to be applied in the determination of bacterial endotoxins (pyrogens) on sterilized or sterilizable healthcare products, components, or raw materials. Specifies general criteria to be . It also provides a uniform basis of evaluating, in a laboratory, the ability of shipping units to withstand the distribution environment. Endotoxin methodologies .January 1, 2011. Bacterial endotoxins—Test methods, routine monitoring, and alternatives to batch testing .
AAMI ST72
Association for the Advancement of Medical Instrumentation [aami] Add to Alert. Several sections in this document have been restructured and extended or changed from ANSI/AAMI ST72. Les endotoxines des bactéries gram-négatives sont la . Bacterial endotoxins—Test methods, routine monitoring, and alternatives to batch testing. NOTE Although the scope of this standard is limited to . Google Scholar. Key Updates While the revised . The committee is working on development of AAMI/CN27,AAMI ST72-2011 《细菌内毒素 - 测试方法 常规监测和批次测试的替代方法》等效标准词汇表委员会代表文献引言1范围2规范性引用文件3定义4质量管理体系要素5非致热性标签6产品单位选择7技术选择8方法的验证9技术的使用10批次测试的替代方法A内容A - 细菌内毒素 . 标准与法规 . This document specifies general criteria to be applied in the .ANSI/AAMI ST72:2011 (ANSI/AAMI ST 72:2011) Bacterial endotoxin - Test methods, routine monitoring and alternatives to batch testing. Add to cart AAMI TIR76:2021 . Specifies general criteria to be applied in the determination of bacterial endotoxins on or in medical devices, components, or raw materials using bacterial endotoxin test methods.In Electrical Power Systems and Industrial Automation, ANSI Device Numbers can be used to identify equipment and devices in a system such as relays, circuit breakers, or . Specifies general criteria to be applied in the determination of bacterial endotoxins on or in medical devices, components, or raw materials using bacterial endotoxin test methodology.