Astepro allergy fda approval

Step 4: Tilt your head downward toward your toes.spray and Children’s Astepro Allergy (azelastine hydrochloride) nasal spray, 205.
FDA Issues Guidances on Food Allergen Labeling Requirements
Approval: 1996 ---- .Astepro Allergy is different from both Astelin ® (azelastine HCl) 0.* *Applies to first dose only. Astepro ® Allergy is the same strength as prescription Astepro ® (azelastine HCI) 0.
Bayer And Meda Sue Apotex
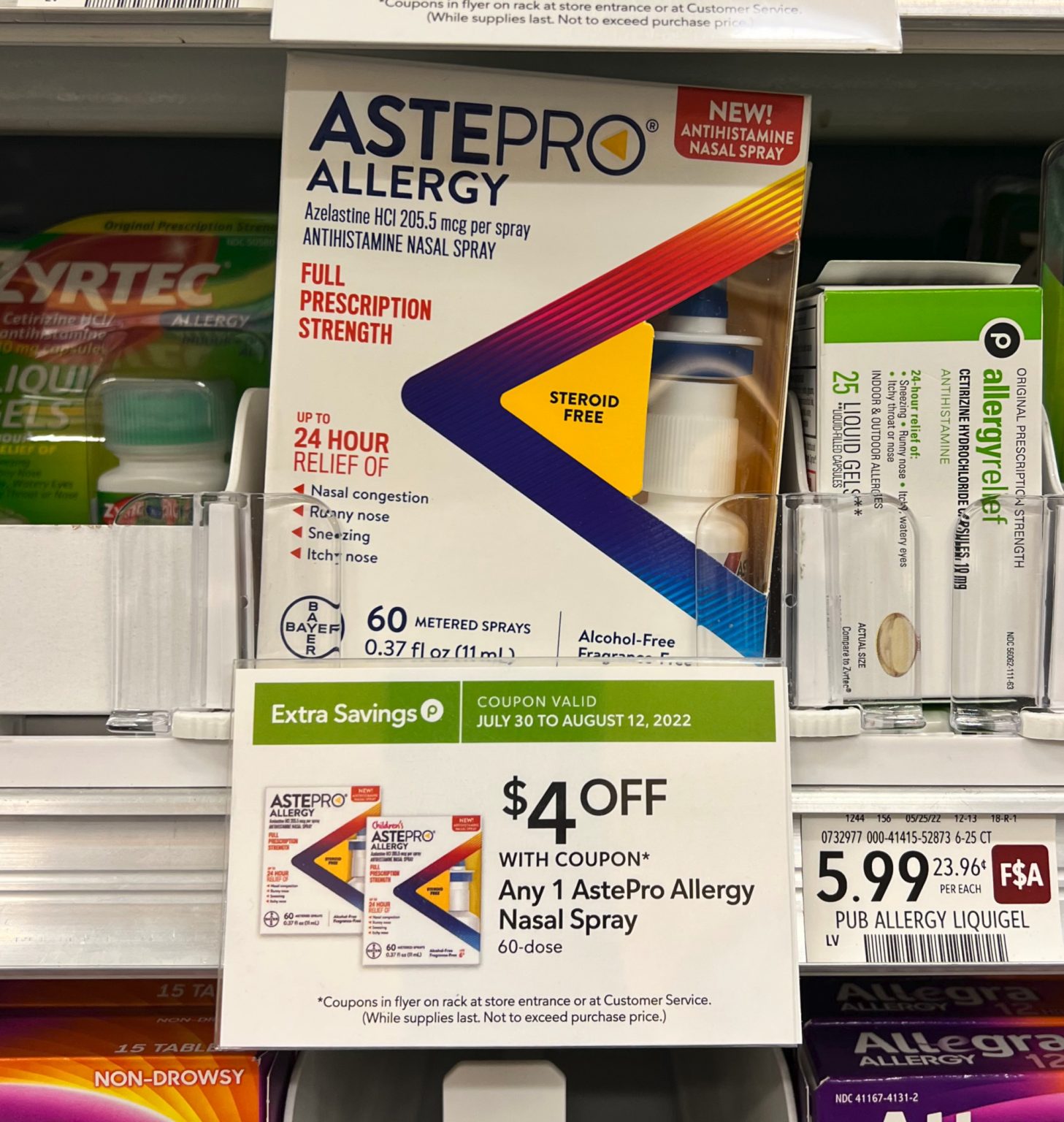
Food and Drug Administration (FDA) has approved Astepro® Allergy (Azelastine .5g bottle, which depending on your dose, could last up to one month.The Food and Drug Administration (FDA) has approved a prescription to over-the-counter (OTC) switch for Astepro ® Allergy (azelastine hydrochloride nasal spray, . First Antihistamine Nasal Spray FDA-Approved for Over-the-Counter Use.15%, a nasal antihistamine, for the relief of symptoms associated with seasonal and perennial allergic . patients won't need a prescription for a nasal spray to relieve. Astepro ® provides full-prescription-strength relief of nasal congestion, runny and itchy nose, and sneezing for up to 24 hours.
Bayer United States of America
Thanks to a new FDA green light, U.The price of Nasacort AQ (Triamcinolone) nasal spray averages around $18 for a 16.10% Nasal Spray 137 mcg (Rx approval in 2008 .
Manquant :
asteproThe Current Food Allergen Landscape
5 mcg per spray and Children’s Astepro Allergy (azelastine hydrochloride) nasal spray, 205.FDA approval history; Drug class: nasal antihistamines and decongestants; Breastfeeding ; Patient resources. Astelin, Astepro Allergy. FDA Approves Astepro Allergy Nasal Spray for OTC Use.
FDA Approves a Nasal Antihistamine for Nonprescription Use
Step 7: Put the spray tip about 1/4 to 1/2 inch into the other nostril.Reference ID: 5280238
Astepro Allergy (azelastine) FDA Approval History
Astepro ® starts to work in 30 minutes.June 17, 2021 - The FDA announced the approval of partial over-the-counter (OTC) use of Bayer’s Astepro Allergy and Children’s Astepro Allergy (azelastine) 0.
Astepro: Package Insert
15% Nasal Spray, 205.
Bayer lands FDA approval for OTC allergy nasal spray Astepro
Officials with the FDA have approved azelastine hydrochloride 0.Bayer claims it is the first over-the-counter, steroid-free antihistamine allergy spray available in the US.137 mL solution containing 205.10% Nasal Spray 137 mcg (Rx approval in 2008). OTHER REVIEW(S) Labeling Review for ASTEPRO® Allergy and Children’s ASTEPRO® Allergy - Addendum. The subjects were randomized to .5 mcg per spray . NDA/SUBMISSION TYPE: NDA 213872.On April 23, 2021, the Food Allergy Safety, Treatment, Education, and Research (FASTER) Act was signed into law, declaring sesame as the 9th major food .
Efficacy
Other allergy sprays take hours. Related treatment guides. It is different than both Astelin® (azelastine HCI) Nasal Spray 137 mcg (Rx approval in 1996), and Astepro (azelastine HCI) 0. The FDA initially approved it in 2008 as a . 药品注册申请号:213872. See full prescribing information for ASTEPRO ® ASTEPRO (azelastine hydrochloride) nasal spray Initial U.
Nasal Allergy Rx to Go OTC

Manquant :
astepro According to Bayer, the approval is the FDA’s first for a steroid-free, antihistamine .Meda won FDA approval for a prescription version of Astepro, a revised formulation of the azelastine active ingredient also used in Astelin, in 2008.15%, the following adverse reactions have been identified.FDA approves nasal spray for nonprescription use to relieve allergies.15%) as an over-the-counter (OTC) product for the temporary relief of nasal congestion, runny nose, sneezing and itchy nose due to hay fever or other upper respiratory allergies. This approval is considered a first-of-its-kind moment because, while it is now approved for OTC use in older children and adults, Astepro will still be available by prescription only for children younger than 6, the .Astepro® Allergy and Children’s Astepro Allergy (azelastine) – New partial over-the-counter approval • On June 17, 2021, the FDA announced the approval of partial over-the-counter (OTC) use of Bayer’s Astepro Allergy and Children’s Astepro Allergy (azelastine) 0.Step 3: Blow your nose to clear your nostrils.5 mcg (Rx approval in 2009), and is the maximum strength of azelastine HCI available as a prescription or over the counter.10% Nasal Spray 137 mcg (Rx approval in 1996), and Astepro (azelastine HCl) 0. The FDA has approved Bayer’s nasal spray Astepro (azelastine hydrochloride) for nonprescription use for seasonal and perennial allergies. SUBMISSION DATES: August 20, 2020 April 9, 2021 April 27, 2021 April 30, 2021 May 14, 2021 May 20, 2021.15% dose is available OTC. Thus, if you are in need of regular allergy relief, it's clear that brand-name . This new drug application provides for the use of Astepro Allergy (azelastine hydrochloride) nasal spray, 205.15%) for seasonal and perennial allergic rhinitis—commonly known as allergies—for adults and children six years of age and.商品名:astepro allergy,活性成分:azelastine hydrochloride,申请号:213872,申请人:bayer hlthcare . The price of Astepro (Azelastine) nasal spray is about $140 for a 30ml bottle, which also typically lasts one month.
The FDA has approved Astepro (azelastine HCl nasal spray, from Meda) 0.
FDA approves antihistamine nasal spray for over-the-counter use
15%), manufactured by Bayer, for over-the-counter (OTC) use to provide temporary relief of nasal congestion, runny nose, sneezing, and itchy nose due to hay fever or other upper respiratory allergies in individuals who are at least six years of age.15% (Astepro Allergy; Bayer) as an OTC product for the temporary relief of nasal congestion, runny nose, sneezing, and itchy nose associated with upper respiratory allergies such as hay fever.
ASTEPRO ALLERGY-美国FDA药品数据库-药物在线
September 2, 2009. FDA has approved Astepro ® Allergy (azelastine hydrochloride nasal spray, 0. 12 Astepro dosing can vary: the 0. Full prescription strength now available without a prescription. allergies Consumer health FDA approvals OTC.On 17 June 2021, the US Food and Drug Administration (FDA) announced approval of a new nasal antihistamine (Astepro Allergy and Children’s Astepro Allergy) for nonprescription use. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.5 mcg per spray.Published July 23, 2021.By Angus Liu Jun 18, 2021 10:15am.15%, for the temporary relief of nasal congestion, runny nose, sneezing and itchy nose due to hay fever or other upper respiratory allergies in adults and children 6 years of age and older. INDOOR & OUTDOOR.APPLICATION NUMBER: 213872Orig1s000. If any of these . Approval is a first-in-class and was enabled by the prescription to nonprescription switch process.
FAQ: Answers to Top Questions about Astepro® Allergy OTC
Starts working in 30 minutes, while other allergy sprays may take hours*. In clinical trials, 94% of patients receiving Astepro® reported no bitter taste2,6. ACTIVE INGREDIENTS: Azelastine hydrochloride, 0.With the FDA’s approval, Astepro® Allergy becomes the first and only steroid free, antihistamine nasal spray for allergies available OTC in the United States for . Adverse reactions . It is a “partial switch” because the allergy .
Reference ID: 5280238
A bitter taste in the mouth, drowsiness, tiredness, nasal discomfort, sneezing fits, cough, nose bleeds, runny nose, dry mouth, headache, or red eyes may occur.1% and ASTEPRO 0.5 mcg per spray for the temporary relief of . Astepro prescribing information; Other brands.5 mcg of azelastine . (Mon–Fri, 9AM–5PM EST) 2021 Bayer.
Astepro Allergy and Children’s Astepro Allergy (azelastine)
Allergic Rhinitis; Further information. Hold bottle upright and aim the spray tip toward the back of the nasal . 4813202 ASTEPRO First Antihistamine Nasal Spray FDA-Approved for Over-the- Counter Use . Today’s OTC approval of Astepro® Allergy will provide more .During the post approval use of ASTEPRO 0. “With Astepro® Allergy, allergy sufferers can get relief of allergy nasal symptoms in an over-the-counter treatment option without .Astepro ® is a first-of-its-kind, steroid-free nasal antihistamine spray that starts working in 30 minutes for fast-acting 24-hour relief.November 29, 2022. The complaint filed on Thursday asks the US District Court for the District of Delaware to delay the FDA’s approval of Apotex’s generic drug until the final Astepro patent expires in 2028. This non-steroidal nasal spray is good news for the 60 million people in the U. Professional resources.

The following packages are also available for this product: NDC Package Code Package Description; 0280-0065-02: 120 CARTON in 1 BOTTLE / 1 SPRAY, METERED in 1 CARTON: 0280-0065-03: 120 .
Astepro® Allergy Nasal Antihistamine Spray
It is different than both Astelin . First and only 24-hour steroid-free allergy spray. Other Product Packages.Bayer announced today that the FDA has approved Astepro Allergy (Azelastine HCI . 产品号 商品名 活性成分 剂型/给药途径 规格/剂量 参比药物(rld) 生物等效参考标准(rs) 治疗等效 .Astepro® Allergy also has the same improved taste profile as prescription Astepro®2,4.FDA approves a nasal antihistamine for nonprescription use.June 17, 2021 – The U. Food allergy treatment is undergoing a paradigm shift with new therapies emerging, including the recent FDA approval of omalizumab — but without .Labeling Review for ASTEPRO® Allergy and Children’s ASTEPRO® Allergy.1% dose will remain available by prescription only, and the 0.