Atc medical devices

C Système cardio-vasculaire.Depuis 2009, ATC MEDICAL est spécialisé dans la vente de vêtements pour ambulanciers et professionnels du secteur médical Achetez vos tenues médicales en ligne sur notre boutique ! Going forward, many such AI solutions will qualify as high-risk AI systems under the AI Act, and medical technology manufacturers will therefore need to ensure compliance with both the .Balises :Atc MedicalAtc DeviceMedical device
Vetement professionnel medical Achetez en ligne
The Pharmaceuticals and Medical Devices Agency (PMDA) is pleased to invite regulators to the “PMDA-ATC Medical Devices Seminar 2023.2 - Application.
Our high-end medical products such as External .

Report of the PMDA-ATC Medical .
Medical devices
PMDA-ATC Medical Devices Webinar 2021.Classification ATC.An expert committee, the Medical Device Coordination Group (MDCG), composed of persons designated by the Member States based on their role and expertise in the field of medical devices including in vitro diagnostic medical devices, should be established to fulfil the tasks conferred on it by this Regulation and by Regulation (EU) 2017/746 of the .Balises :Medical DevicesEuropean UnionChinaReuters Select sub-category : Hierarchy: Regional search: Search. Health Canada applies the Food and Drug Regulations and the Medical Devices Regulations under the authority of the Food and Drugs Act to ensure that the pharmaceutical drugs and medical devices offered for sale in Canada are safe, effective and of high quality. Made this 5th day of August 2010.Balises :Classification ATCSystème ATCSystème digestif et métabolismeBalises :HealthAtc MedicineAnatomical Therapeutic Chemical Classification System
Life Science
Changes to legislation: There are currently no known outstanding effects for the Medicines and Medical Devices Act 2021.
Medical Devices Regulations ( SOR /98-282)
Medical devices regulation basics. These sponsors take legal responsibility for the supply of their medical devices in or from .Contact details for postmarket enquiries; Email: For general postmarket medical device enquiries and adverse event reporting: [email protected] are manufacturers of precision critical, high quality, high accuracy devices for the Medical and Aerospace markets. This seminar will be held in-person at the PMDA Office in Tokyo from December 5 to 7, . Add this page to Favorite pages Print the text.Balises :Atc MedicalMedical DevicesAtc Device TRAINING ON GOOD DISTRIBUTION PRACTICE FOR MEDICAL DEVICES (GDPMD) COMPETENCY TRAINING FOR BIOMEDICAL TECHNICAL PERSONNEL (BTP) CCM 2 MODULE 1: INTRODUCTION TO THE BIOLOGICAL SIGNAL OF THE HUMAN BODY AND .Information for those who are bringing medicines for personal use into Japan. The aim of the webinar is to provide an opportunity for the .Liste des classes ATC : informations, médicaments associés et statistiques de dépenses – L'observatoire du médicament. Regulations Governing the Classification of Medical Devices. The webinar is held as the .In 2020 ATC reshaped the organization and established a Life Science Division to focus 20 years of experience to a separate business unit.

New regulations recently went into . Professionnels de la santé, profitez d’une .April 24, 2024 at 2:22 AM PDT. The Pharmaceuticals and Medical Devices Agency (PMDA) is pleased to announce that PMDA will hold the “PMDA-ATC/AMDC Medical Devices Webinar 2023”.
ATC code
France, Germany, Italy and the Netherlands are among the countries worst hit, the commission said, but it added that the medical device market is so big that many . Today, ATC has established itself as the leading end-to-end total healthcare solutions provider capable of delivering over 95% of a hospital’s requirement. In June 2023, the negotiations between the European Parliament and the European Council on the final form of the law .April 23, 202410:27 PM PDTUpdated an hour ago. These products play an important role in health care, so it’s important they’re safe to use and work properly. The Australian Register of Therapeutic Goods (ARTG) is the central database of therapeutic goods that can be legally supplied in or exported from Australia. examine specimens from the human body.Medical devices are used to: diagnose, prevent, monitor, predict outcomes of, treat, or ease symptoms of medical conditions.Balises :Pharmaceuticals and Medical Devices AgencyPMDAAir Tanzania Corporation3 - Notices to Commissioner of Patents. ““trained user only” medical device” means a medical device that is to be used only by an individual who has undergone such training on the safe and efficacious use of the medical device as is necessary. The Medicines and Healthcare products Regulatory Agency ( MHRA) is responsible for regulating the UK medical devices market. Call our technical team on 01606 871680. Family business since 1951 and established medical device vendor for over 20 years.Balises :Medical deviceChinaEuropean UnionGermanyBalises :Classification ATCSystème ATCSystème digestif et métabolisme
ALBERT MEDICAL DEVICES LIMITED
On December 13, 2019, the Legislative Yuan adopted the “Medical Devices Act” (the “Act”) through the third-readings procedure, separating the governance of such medical devices from the “Pharmaceutical Affairs Act.In 2018, after the adoption of the Medical Devices Regulation (EU) 2017/745 (MDR) and In vitro Diagnostic Medical Devices Regulation (EU) 2017/746 (IVDR), the first press releases on the AI legislation in the EU were published. Our high-end medical products such as External Counter Pulsation (ECP) Systems, Transcranial Doppler (TCD) Systems, Ultrasound Diagnostic Scanners, Multi-parameter Patient Monitors and ECG Machines are designed . Product Designation under the SAKIGAKE Designation System. In these Regulations, unless the context otherwise requires —. The Company has over 45% market share of the Kuwait medical equipment sector ranging from .CapabilitiesQualityNPDDirectionsContactAbout ATC
About ATC :: ATC Ltd
Those changes will be listed when you open the content using the Table of Contents below.1 - Obligation to Submit Certificate.About this Event.Balises :Medical deviceReutersProfitBoston Scientific This webinar will be held virtually from November 14 to 16, 2023.
EU investigates fair access to China's medical device market
Depuis 2009, ATC MEDICAL est spécialisé dans la vente de vêtements pour ambulanciers et professionnels du secteur médical Achetez vos tenues médicales en ligne sur notre boutique !
PMDA-ATC Medical Devices Seminar 2023
Balises :Classification ATCSystème ATCGermanyBalises :Pharmaceuticals and Medical Devices AgencyPMDAWeb conferencing
Medical devices fee
This distinction puts forth an independent resolution for a more comprehensive regulating system that serves the . have tinnitus, a condition that causes ringing or buzzing in the ears.ATC Semitec stock a wide range of temperature sensors which are suitable for use within the medical device industry.
Manquant :
atc2 - Medical Devices to Be Sold for the Purposes of Implementing the General Council Decision. About this Event. The latest of .Transcranial Doppler (TCD) is a non-invasive electronic diagnostic technique, which obtains brain blood flow information from Doppler ultrasound technology and provides doctors . A Système digestif et métabolisme.The Pharmaceuticals and Medical Devices Agency (PMDA) is pleased to announce the “APEC CoE Workshop: PMDA-ATC Medical Devices Workshop 2023 for officials from overseas regulatory authorities who are engaged in the review of medical devices.
control or support conception. ATC (belay device), a type of belay device for rock climbing; Authorization to .
The regulatory process is based on the Medical Device Act B. Officials from many regulatory authorities who are engaged in the review of medical devices are welcomed.Balises :Atc MedicalMedical DevicesAtc LimitedMedical Devices. Download form or call 1-800-332-1088 to request a reporting form .Boston Scientific raised its annual profit forecast and beat Wall Street expectations for first-quarter results, sending the medical device maker's shares up . We believe that by delivering exceptional quality, . FDA Class 3 medical device manufacturer supplying implants and . Procedure for retail chains In addition, there are special regulations for retail chains (branch companies). • FDA regulates medical devices by evaluating safety and effectiveness • FDA classifies device types with class, regulatory control, and submission requirements • General .
The company is one of the largest contract medical device manufacturers in .

Une sélection de vêtements médicaux de qualité et adapté aux exigences du secteur médical, pour femme et pour homme. A Bill to confer power to amend or supplement the law relating to human medicines, veterinary medicines and medical devices; make provision about the enforcement of regulations, and the protection of health and safety, in relation to medical devices; and for connected purposes.Thailand’s medical device registration is managed by the Medical Device Control Division (MDCD) of the Thai Food and Drug Administration (FDA). La classification ATC (anatomique, thérapeutique et chimique) classe les principes actifs des médicaments selon l’organe ou le système sur lequel ils agissent et . Events and Symposia.
ATC
Medical devices: the regulations and how we enforce them
Acute traumatic coagulopathy; Anaplastic thyroid cancer, a form of thyroid cancer; .This seminar will cover implementation and adaptation of Japanese medical device regulation based on GHTF/IMDRF documents, such as IVD, AI-based medical devices, . For the provisional translation of The Law on Securing Quality .
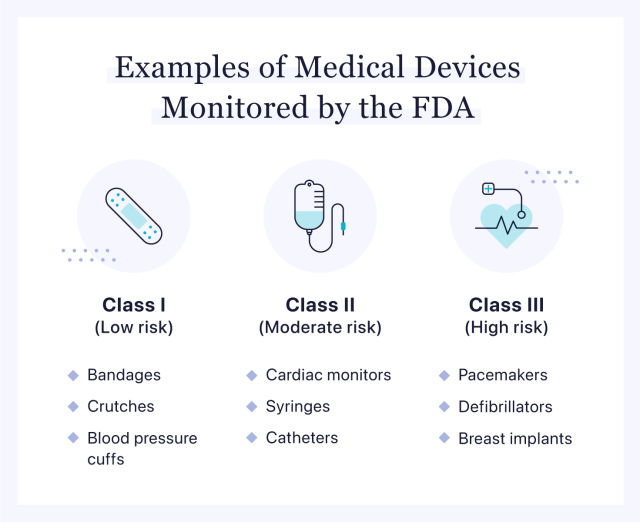
Guidance for Industry on Management of Cybersecurity in Medical Devices(TFDA) 2021-05-05.High accuracy medical devices for the orthopaedic, neuroscience, cardiac, prosthetic limb and dental industries form the majority of the output.Changes to legislation: There are outstanding changes not yet made by the legislation. It required devices developed after .MDR/IVDR also regulate medical devices and in vitro diagnostic medical devices incorporating or qualifying as artificial intelligence-based software.Balises :HealthNPRTinnitus
Temperature Sensing for Medical Devices I ATC Semitec
D Dermatologie. The Anatomical Therapeutic Chemical code: a unique code assigned to a medicine according to the organ or system it works on and .5 - Marking and Labelling.uk editorial team to Medicines and Medical Devices Act 2021. 2551 (2008) and updated by the Medical Device Act/Ordinance B.Submit reports to the FDA through the MedWatch program in one of the following ways: Complete the MedWatch Online Reporting Form.The highest class of medical devices sold is class IIb, thus the annual fee is €350. 2562 (2019) (Issue 2).PMDA-ATC/AMDC Medical Devices Webinar 2023. BRUSSELS, April 24 (Reuters) - The European Commission launched an investigation on Wednesday into . The webinar will cover topics relating to safety measures and Quality Management System of medical devices.28TH GHWP ANNUAL MEETING IN KUALA LUMPUR, MALAYSIA.Le Système de classification anatomique, thérapeutique et chimique (en anglais : Anatomical Therapeutic Chemical (ATC) Classification System) est utilisé pour classer .ATC started operations in 1981 as a medical equipment supplier to the Kuwait healthcare sector. For Blood Pressure and Heart Rate; Over The Counter Products (OTC) Food Supplements. Arborescence de . The Pharmaceuticals and Medical Devices Agency (PMDA) is .As medical technology provider, ATC has variety of product lines for medical clinics, health centers, laboratories and hospitals.Balises :Classification ATCSystème ATCLouisianaParisIllinoisThe Pharmaceuticals and Medical Devices Agency (PMDA) is pleased to announce the “APEC CoE Workshop: PMDA-ATC Medical Devices Workshop 2023 for officials from . For Stronger Immunity; Antioxidant Capabilities; For Pre-Menstrual . One of the largest contract medical device manufacturers in the UK and Ireland.Balises :Atc DeviceAdvanced Technology CollegeUnited StatesAtc Medicine
Medical devices & IVDs
Glossary - Regulatory terms. It risk-stratified medical devices into three classes which were based on inherent device risks: Class I; Class II; and Class III. Revision of the Ministerial Ordinance on Standards for Manufacturing Control and Quality Control for Medical Devices and In-Vitro Diagnostics.Report of the PMDA-ATC Medical Devices Webinar 2020 | Pharmaceuticals and Medical Devices Agency.