Atoms contain mostly empty space
surrounded by empty space and then a layer of electrons to form the outside of the atom.
Once all the charges are out of the way the atoms (now balls of neutrons) are free to collapse .Temps de Lecture Estimé: 3 min
Ask Ethan: How can matter be mostly empty space?
Why can’t we see .
Who discovered that atoms are mostly empty space?
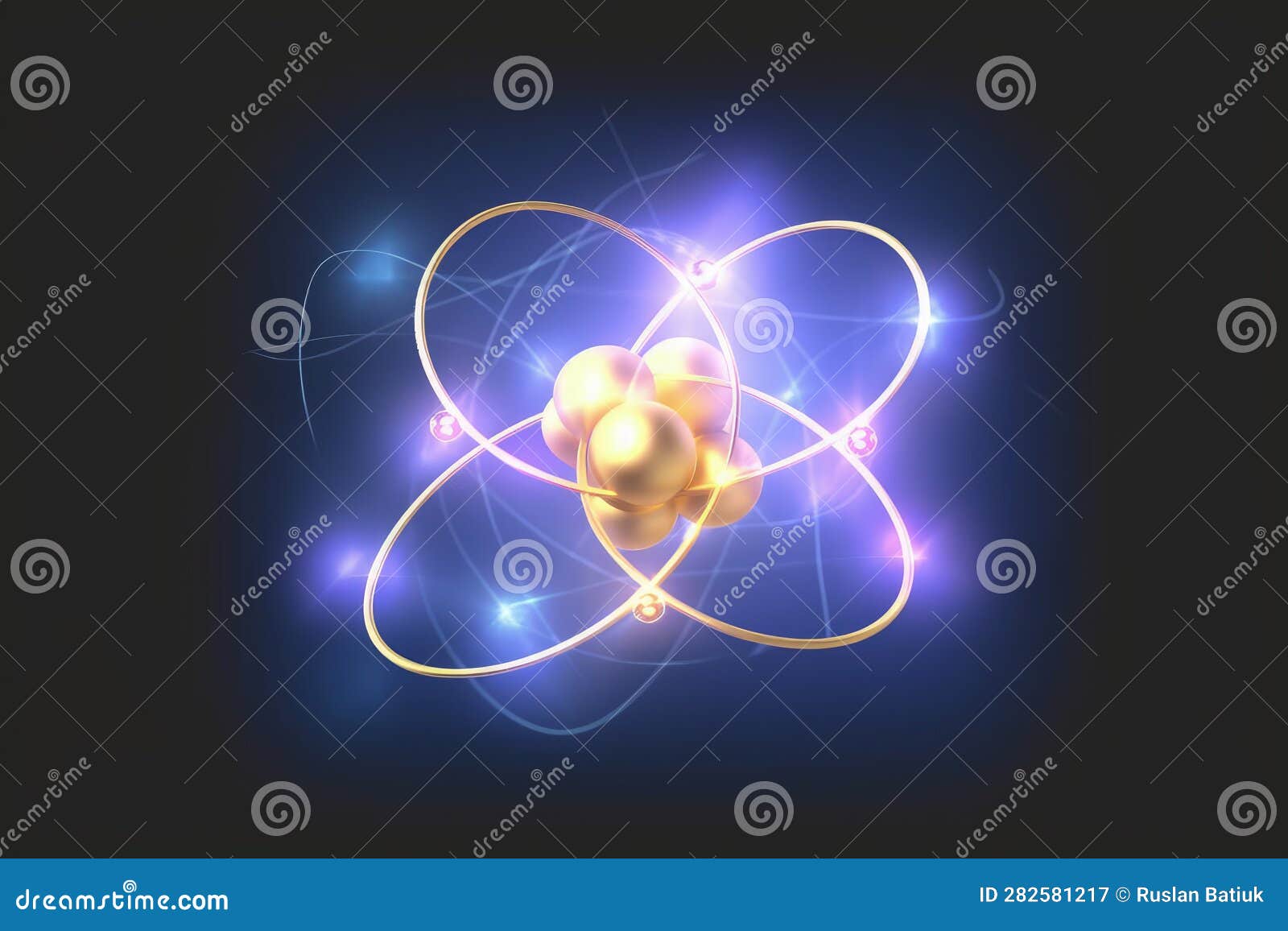
The electrons make up a tiny proportion of the mass of an atom, while the nucleus makes up the rest. 81 people found it helpful. If we consider an atom to that of a football stadium, the nucleus would be about the size of a pea. The atom was mostly empty space.Atoms are mostly empty space Atomic Theory: Part 1.9999999% of Your Body Is Empty Space : ScienceAlertsciencealert.This electron-nucleus overlap makes possible the effect of electron capture, where a proton in the nucleus can react with an . He sent a beam of alpha particles toward .If atoms are mostly empty space, why do objects look and feel solid? by Roger Barlow, The Conversation. The mass of our . If an atom were about as big as a baseball stadium, the nucleus would be the size of a pea in the very center and the electrons would be somewhere on the outside edge. Through this he discovered that there is a small, dense, positively charged nucleus; most of an atom’s mass in the atom’s nucleus; atoms contain mostly empty space; electrons move in empty space in the atom. The gold foil experiment consisted of a series of tests in which a positively charged helium particle was shot at a very thin layer of gold foil.
Discovery of the electron and nucleus (article)
Democritus: He conducted experiments in combining elements.
Ernest Rutherford's Gold Foil Experiment
The electrons revolve in circular orbits about a massive positive charge at the centre.Atoms contain almost all of their mass in a tiny nucleus at the center of the atom (like a marble in a soccer stadium).9999999999996%. This is because, an electron in a low energy level around one . Democritus: Electron paths cannot be predicted. If you touch the table, then the electrons from atoms in your fingers become close to the electrons in the table’s atoms.Atoms are the basic particles of the chemical elements.The electrons in the atom must be orbiting around this central core, like planets around the sun, Rutherford proposed.
Rutherford model
Rutherford: Atoms are small, hard particles. Percent Empty = 99.Atoms are mostly empty space: Some particles are deflected: Positively charged particles in atoms repelled alpha particles : A few particles are deflected by large angles: Atoms contain a relatively small object, with a relatively high mass and charge: All the alpha particles should have passed straight through the gold foil if the plum pudding model . According to quantum electrodynamics, the space is filled by . Buzzing randomly around .
The Universe is Mostly Empty Space
Atoms are mostly empty space This sits right near the top of my list of Unhelpful Science Factoids That Aren't Really So.
If atoms are mostly empty space, why is matter not transparent?
If atoms are mostly empty space, why is matter not transparent? - BBC Science Focus Magazine. The nucleus makes up a tiny proportion of the space occupied by an atom, while the electrons make up the rest.? Schrodinger and Heisenburg. Put another way, if a hydrogen atom were the size of the earth, the proton at its center would be about 200 meters (600 feet) across. Which of the following statements about protons are false? They too have empty space between their nucleons and electrons.Yet the atom, the building block for all materials solid, liquid, gas, and more, found on Earth and beyond, is mostly empty space, with very little volume taken up by . Who said,Electron paths cannot be predicted.
Atoms are mostly empty space, and although I now understand why matter doesn't pass through other matter, why don't photons pass through the empty space of .9999999999996% empty space.Find step-by-step Chemistry solutions and your answer to the following textbook question: Which statements are *consistent* with Rutherford's nuclear theory as it was originally stated? Why? (a) Atomic nuclei are small compared to the size of atoms. Basically, they find that it’s easier for the electrons and protons to fuse together and form neutrons. Surrounding that nucleus are the electrons and .
Which statements are *consistent* with Rutherford's nuclear
Chemist John Dalton proposed .Rutherford's gold foil experiment showed that the atom is mostly empty space with a tiny, dense, positively-charged nucleus. The small positive nucleus would deflect the few particles that came close. Rather, space is filled with a wide variety of particles and fields. The nucleus of the atom is extremely small.Pocket Scholar. Who said,Atoms are uncuttable.As shown in Figure 22. Air molecules are also made up of atoms with a central core of nucleons and electrons spinning around them.Yet the atom, the building block for all materials solid, liquid, gas, and more, found on Earth and beyond, is mostly empty space, with very little volume taken up by substantive particles.

(c) Neutral potassium atoms contain more protons . Credit: Max Rahubovskiy .Atoms were shown to contain mostly empty space. What makes an atom of gold different from an atom of iron is the number of protons, neutrons, and electrons inside it.If atoms are mostly empty space, why do objects look and feel solid? Published: February 16, 2017 5:21am EST.Rutherford's gold foil experiment showed that atoms are mostly empty space, with the positive charge concentrated in a nucleus. Thomson; see history of subatomic .0000000000004%. Add your answer and earn points.By Keith Cooper.If atoms are mostly empty space, why do objects look and feel solid? February 17, 2017.You Are Not Mostly Empty Space - Forbesforbes.My conclusion will be that the answer is no: it is not true that the interior of an atom is mostly vacuum or empty space, and furthermore such an idea conveys a .Outside of the nucleus, an atom is mostly empty space, with orbiting negative particles called electrons whizzing through it. A hydrogen atom is about 99. You're mostly a series of electron clouds, all bound together by the quantum rules that govern the entire Universe. Dalton: Atoms are uncuttable.Why are atoms mostly empty space? By Phil Plait. Its radius is only about 1/100,000 of the total radius of the atom.9999999 percent empty space. neutron and proton electron. All the elements in the Periodic Table are made from different atoms, and the structure of these atoms results in a .Due to the Space inside Atoms, You Are Mostly Made up of Empty Space - Interesting Engineering.Who discovered that the atom is mostly empty space? Flexi Says: Ernest Rutherford discovered the nucleus of the atom in 1911. Here's the deal. It’s true that a large percentage of the atom’s mass is concentrated in its tiny nucleus, but that does not imply that the rest of the atom is empty. Professor Brian Cox is a physicist in England, very well-known there as a popularizer of science.Temps de Lecture Estimé: 4 min
If atoms are mostly empty space, why do objects look solid?
Later we learned that an atom was mostly empty space, which is the knowledge that led us to our current understanding of the atom that has a nucleus where the protons and neutrons sit. Far from the nucleus are the negatively charged electrons.If atoms are mostly empty space, why do objects look and . Based on these results, Rutherford proposed the . The discovery of the make-up of the nucleus (protons and neutrons) came . One can picture them to be like the solar system with the sun at the center and the planets revolving around . Loved by our community. Add answer +5 pts.3, the dense nucleus is surrounded by mostly empty space of the atom, an idea verified by the fact that only 1 in 8,000 particles was scattered backward. However, this metaphor happens to be wrong.

empty space - Physics Stack Exchangephysics. Chemist John Dalton proposed the theory that all matter and objects are . But how is it possible that we . (b) The volume of an atom is mostly empty space.The volume of an atom is mostly empty space. As I previously wrote in a story for the particle physics publication Symmetry , the size of an . Rutherford carried out a fairly simple calculation to find the size of the nucleus, and found it to be only about 1/100,000 the size of the atom. Atoms make up all of the matter in our universe and they are amazingly tiny- you can line up 200,000 of them along the point of a pin. The reasons for this are .The Rutherford atomic model was correct in that the atom is mostly empty space. Fun fact: if gold atoms were the size of these cannonballs, one . Who conducted experiments In combining elements? Dalton.There is no empty space around a nucleus, as in Bohr's superseded model.Atoms are not mostly empty space because there is no such thing as purely empty space. Electrons have been known since the late 19th century, mostly thanks to J. The protons and neutrons form the atom’s central nucleus. His model explained why most of the α particles passed straight through the foil.999999% empty space.Why doesn't matter pass through other matter if atoms are 99.Second, there is the table of science.
If atoms are mostly empty space, why do objects look and feel solid?
Electrons have . published 30 October 2022. By Roger Barlow - University Huddersfield. Who said,Atoms are small, hard particles.Atoms contain mostly empty space. klynn2002 is waiting for your help.orgRecommandé pour vous en fonction de ce qui est populaire • AvisBut it might humble you to know that all of those things – your friends, your office, your really big car, you yourself, and even everything in this incredible, vast Universe – are almost entirely, 99.

Who said,Electrons travel I'n definite paths. What are Nucleus Made of .It feels solid because of the dancing electrons.All but the simplest type of atoms, and we’ll specify that notable exception shortly, contain varying numbers of all three of these three subatomic particles.Most of the atom is empty space.This was all on display recently when he hosted a great segment on the BBC's show A Night With The Stars, where he simply and effectively demonstrates why .Atoms are mostly empty space, so why doesn’t the world collapse and then blow up? Atoms make up all of the matter in our universe and they are amazingly tiny- you can line up 200,000 of them along the point of a pin.comRecommandé pour vous en fonction de ce qui est populaire • Avis
A lot of empty space
The space surrounding the . If atoms are mostly empty space, why do objects look solid? An atom is composed of 99.
Atomic Theories :/ Flashcards
Inside your body, you aren't mostly empty space.
Why are atoms mostly empty space?
If atoms are mostly empty space, why do objects look and feel solid? February 16 2017, by Roger Barlow.It is mostly emptiness with numerous, sparsely-scattered electric charges rushing about with great speed. The figure below shows these parts of the atom.Even though we perceive matter as solid, the individual atoms are to a large extent empty: Even a diamond consists mostly of empty space, says Fritzsch. Protons have about the same mass as .Addressing the fact that matter is mostly empty space, if you really squeeze matter you’ll find that the electrical forces can no longer hold atoms apart. Atoms are composed of positively charged protons, neutral neutrons, and negatively charged electrons. This is something that is intangible. 20th Century: Electrons travel in definite paths.Science & technology news. The gold atoms in the ingots shown in Figure 3-3 pack together in a manner that is similar to that seen in the stacking of cannonballs.Most of an atom is empty space.The space inside the atom is just that, empty space, i.
Rutherford: His . The atom is a crazy soup of electrons .Temps de Lecture Estimé: 4 min As the electrons in one atom get close enough to the nucleus of the other, the patterns of their dances change. Most of the mass is in the nucleus, and the nucleus is positively charged. He realized this because most of the alpha particles passed straight .
It depends on a half-assed understanding of the Bohr model which is wrong in a lot of ways, but is still taught because it is a easy stepping stone toward a real understand of atoms. mass of ~ 1 amu mass of ~1/2000 amu. Teacher Support [BL] [OL] Students should recognize two .