Aufbau principle explained

21M subscribers. (b) This diagramrepresents the .The Aufbau principle states that electrons will first fill the lowest energy electron shells in a neutral atom. State and explain the Aufbau principle, write electronic configuration of C,Cr,F e,Cu. In general, an electron will occupy an atomic orbital with the lowest value of n, l,ml n, l .In this Chemistry video in Hindi for class 11 we explained aufbau principle for electronic configuration of different atoms. This is a key point when teaching Physical chemistry. The Pauli Exclusion Principle states that, in an atom or molecule, no two electrons can have the same four electronic quantum numbers.The Aufbau Principle states that electrons will fill in an atom in a specific order. For example, the 1s subshell is filled before the 2s subshell is occupied. Explain Aufbau Principle. The term comes . Les électrons se . We’ve all seen and use the so-called Aufbau Diagram (Figure 1). This means if one electron is assigned as a spin up (+1/2) electron, the other .Alcohol Phenol and Ethers Full Lecture https:/.
Manquant :
explained It models atomic orbitals as “boxes” of fixed energy into which at most two electrons can be placed. In general, an electron will occupy an atomic orbital with the lowest value of n, l,ml n, l, m l, in that .Demystifying Aufbau Principle in Chemistry • Aufbau Principle Explained • Discover the key concept in chemistry where electrons occupy orbitals from lowest t. It is important to note that there exist many exceptions to the Aufbau principle such as chromium and copper.Introduction to electron configurations.Before continuing, it's important to understand that each orbital can be occupied by two electrons.The Aufbau (German: “building up, construction”) principle is sometimes referred to as the “building-up” principle.conquerchemistry. Atomic theory can be complex and difficult, but this principle provides a simple set of rules that can explain the electron configurations of the vast majority of elements. Aufbau is a German word meaning building up or construction. Rule 1 (Aufbau Principle): Electrons occupy the lowest-energy orbitals possible, starting .The Aufbau principle states that an electron occupies orbitals in order from lowest energy to highest. Todd Helmenstin. The electrons gather around the nucleus in quantum orbitals following four basic rules called the Aufbau principle . Bala's Chemistry BALANAGA KARTHIKThe Aufbau principle is used to predict the electronic configurations of atoms, and accordingly explain the layout of the periodic table, and how the electrons are arranged from low to high energy levels. The Aufbau principle (from the German Aufbauprinzip, which means building-up principle ), also called the Aufbau rule, states that in the ground . Electron configurations describe where electrons are located around the nucleus of an atom. Hund's rule states that orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron and that each of the single electrons must .The aufbau principle says that the arrangement of electrons in an atom - the electron configuration - is best understood if it is built from the ground up.
Google Classroom.
Aufbau Principle: Statement, Example, and Diagram
‘Aufbau’ is a german word that means construction or .comRecommandé pour vous en fonction de ce qui est populaire • Avis
Aufbau Principle
The Aufbau principle states that electrons will inhabit the lowest-energy orbitals first. Stable atoms have as many electrons as protons in the nucleus.The Aufbau principle originates from the Pauli’s exclusion principle which says that no two fermions (e.
Aufbau Principle: Statement, Example, and Diagram
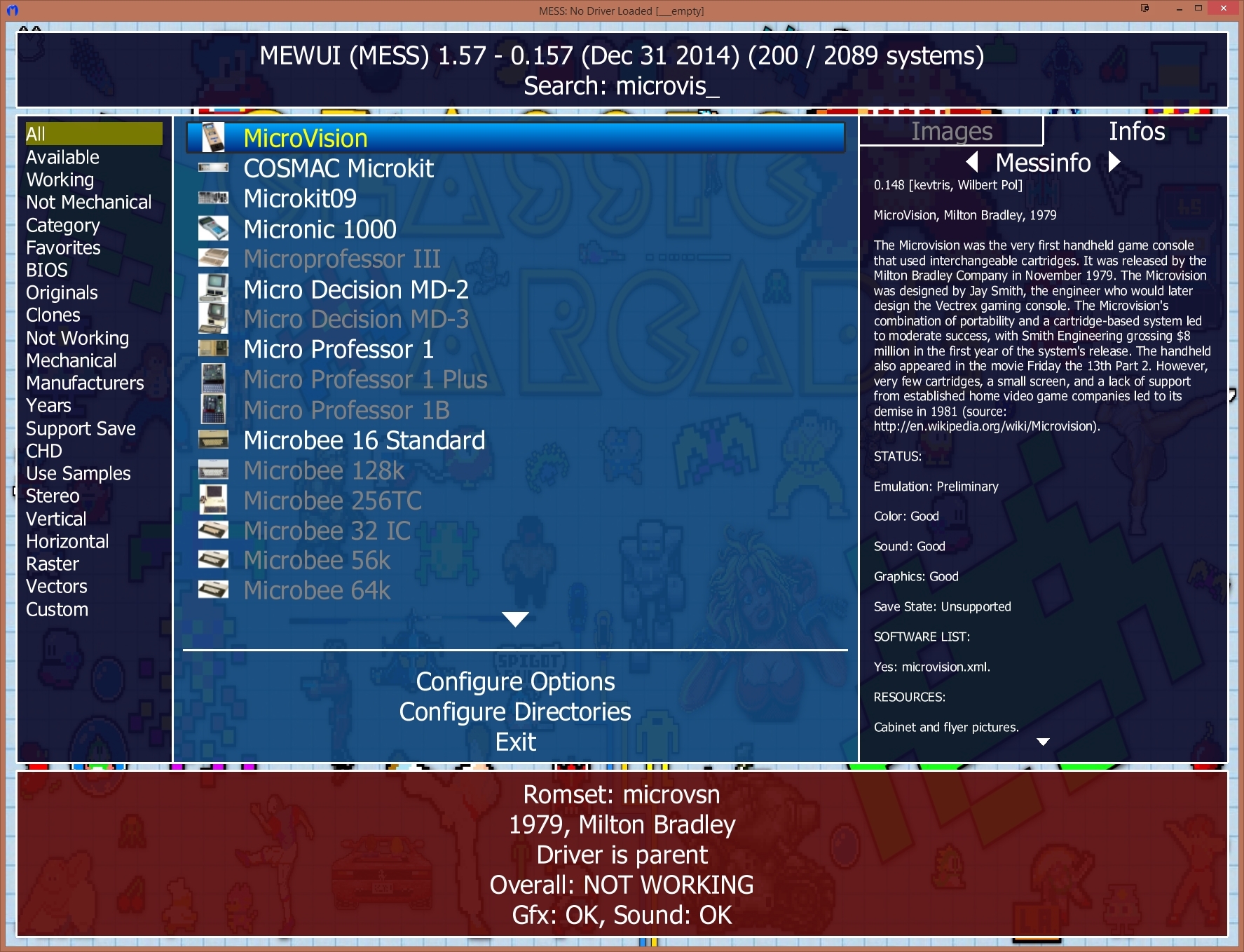
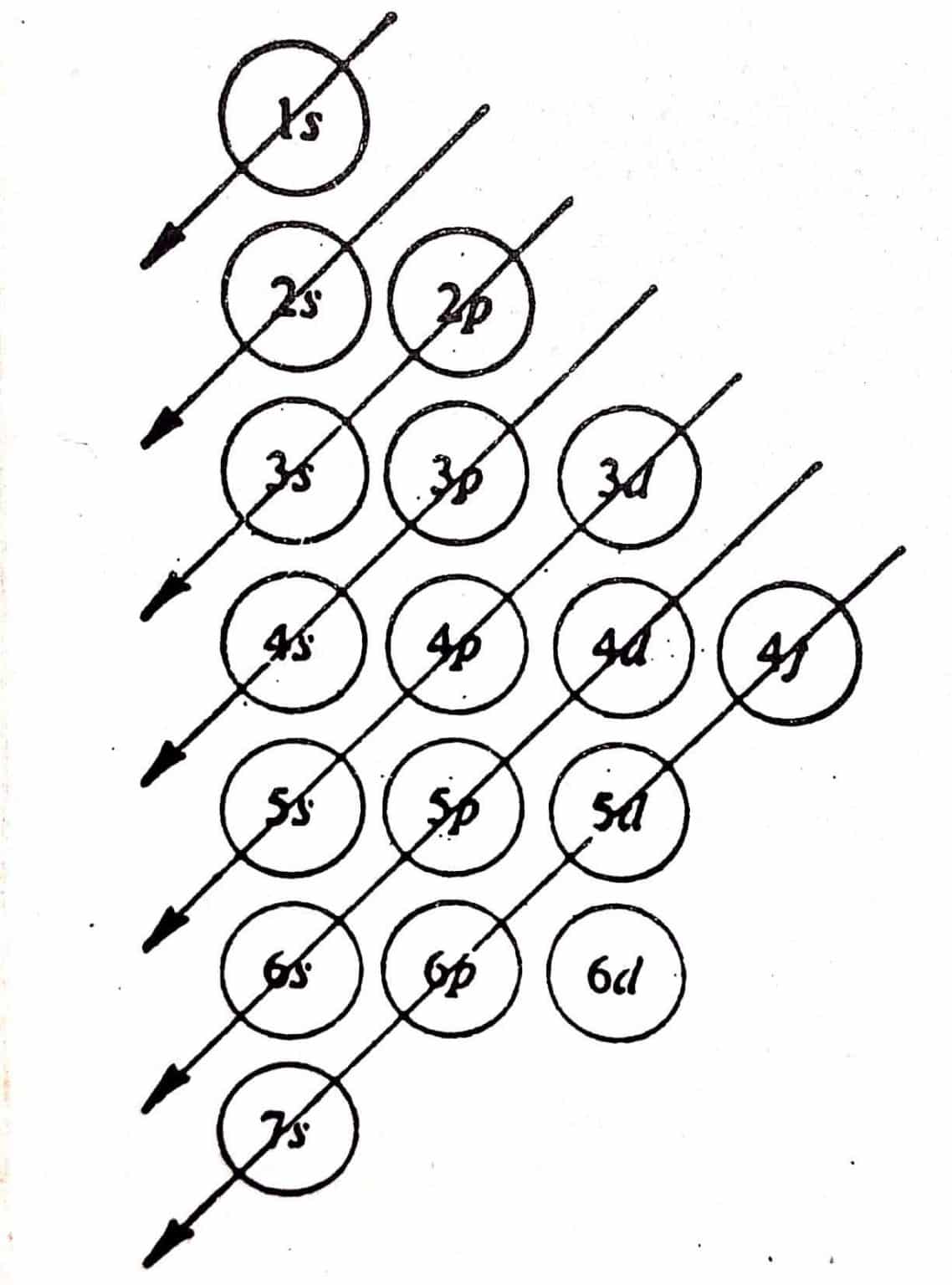
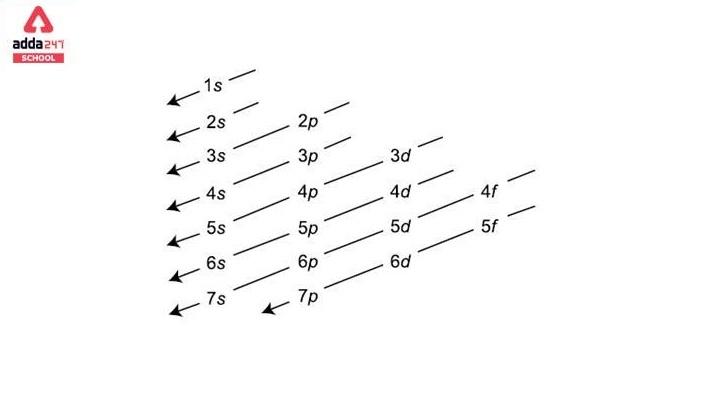
As an orbital can contain a maximum of only two electrons, the two electrons must have opposing spins.com/masterclass📗 Need help with chemistry? Download 12 .The order of filling orbitals - the Aufbau Principle. As seen in Figure above, the energies of the . The aufbau principle, from the German Aufbauprinzip (building-up principle), also called the aufbau rule, states that in the ground state of an atom or ion, electrons fill subshells of the lowest available energy, then they fill subshells of higher energy.
Electron Configuration
First we determine the number of electrons in .
Qu'est-ce que le principe d'Aufbau
Regarder la vidéo13:53#aufbauprincipleintamil,#CBSEchemistryintamilIn this video discussed about the aufbau' s Principle in detail. Pauli Exclusion .
Aufbau principle
Auteur : Sal Khan8: Hund's Rule and Orbital Filling Diagrams is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts.The Aufbau Principle (also called the building-up principle or the Aufbau rule) states that, in the ground state of an atom or ion, electrons fill atomic orbitals of the lowest available energy level before occupying higher-energy levels. The Aufbau principle states some important rules for filling orbitals in an atom. The Aufbau Principle (also called the building-up principle or the Aufbau rule) states that, in the ground state of an atom or ion, electrons fill atomic orbitals of the lowest available energy level before occupying higher-energy levels.The Aufbau Principle (also called the building-up principle or the Aufbau rule) states that, in the ground state of an atom or ion, electrons fill atomic orbitals of the lowest available . Aufbau is a German word meaning . The (n+l) rule can be used to establish the sequence in which the energy of orbitals grows, with . According to Aufbau principle, electrons first occupy the orbitals whose energy is the lowest.Updated on August 31, 2019. A visual representation of the Aufbau Principle and Hund's Rule. It is worth noting that in reality atoms are not built by adding protons and electrons one at a time and that this method is merely an aid for us to understand the end result., electrons) in an atom can have the same set of quantum numbers, hence . This means that electrons can only enter higher-energy orbitals after lower-energy orbitals have been entirely filled. It states that the electrons are filled in the atomic orbitals ( s, p, d, f) in an increasing order of orbital energy level.comAufbau Principle Flashcards | Quizletquizlet. The Aufbau principle requires the atom to adhere to Pauli’s exclusion principle and Hund’s rule, which states that each orbital can initially only hold one electron before becoming doubly occupied (no two electrons in one .The Organic Chemistry Tutor. When writing down an atom's electron configuration, we begin at the lowest energy level and add electrons to higher . General Chemistry Start typing, then use the up and down arrows to select an option from the list. This lecture is about aufbau principle in chemistry and how to find . To determine the electron configuration for any particular atom, we can “build” the structures in the order of atomic numbers. These exceptions can sometimes be explained by the stability provided by half-filled or completely filled subshells. Mis à jour le 31 août 2019. Explain with the help of an example.Aufbau Principle - Definition, Formula and Exceptions - . One energy subshell must be completely filled before a new subshell begins to fill in.The order in which electrons are filled in atomic orbitals as per the Aufbau principle is illustrated below. Electrons fill orbitals from lowest energy orbitals to highest energy orbitals.The Aufbau principle, simply put, means electrons are added to orbitals as protons are added to an atom.
Definition of Aufbau Principle
The principle, formulated by the Danish physicist Niels Bohr about 1920, is an application of the laws of quantum mechanics to the properties of electrons . This chemistry video explains what is .Auteur : Anne Marie Helmenstine, Ph.
Aufbau's Principle, Hund's Rule & Pauli's Exclusion Principle
Aufbau' s Principle
There are a set of general rules that are used to figure out the electron configuration of an atomic species: Aufbau Principle, Hund's Rule and the Pauli-Exclusion Principle.

182K views 2 years ago #chemistry #aufbauprinciple.
The Aufbau principle (video)
The term comes from a German word .The Aufbau principle is based on the idea that the order of orbital energies is fixed—both for a given element and between different elements.Aufbau principle states that an electron occupies an orbital in the order of lowest to highest energy orbital. The order in which an . This assumption is approximately true—enough for the principle to be useful—but not physically reasonable. Aufbau principle fails to explain the configuration of element with atomic number: Q. Beginning with hydrogen, . Write the electronic configuration of Sr2+ in absence of Aufbau Principle. The Aufbau (German for building up, construction) principle is sometimes referred to as the building up .The Aufbau Principle To determine the electron configuration for any particular atom, we can “build” the structures in the order of atomic numbers. Les atomes stables ont autant d' électrons que de protons dans le noyau .Introduction au principe d'Aufbau en chimie.Aufbau principle gives a model for filling the electrons in the empty subshells of an atom in its ground state.
Created by Sal Khan.
Introduction to electron configurations (video)
The presentation of this diagram is largely disconnected from any physical .The Aufbau principle dictates the manner in which electrons are filled in the atomic orbitals of an atom in its ground state.

The Aufbau principle is a crucial chemistry principle for determining an atom’s electron configuration in its ground state.Aufbau principle explained.
Aufbau Principle
By following this rule, we can predict . We imagine that as you go from one atom to the next in the . State True or False. ' Aufbauprinzip ' is a German noun; it means ' construction principle .Aufbau principle-concept of orbitals and their Quantum numbers.🎯 Want to ace chemistry?

Aufbau principle, (from German Aufbauprinzip, “building-up principle”), rationalization of the distribution of electrons among energy levels in the ground (most stable) states of atoms. Define Aufbau Principle. 527K views 6 years ago New AP & General Chemistry Video Playlist. Applying Aufbau principle, write the electronic configuration of element with Z=21. Skip to main content. According to this principle, electrons first fill up the lower . In all atoms, the orbital is always the first orbital to be filled with electrons.
Pauli Exclusion Principle
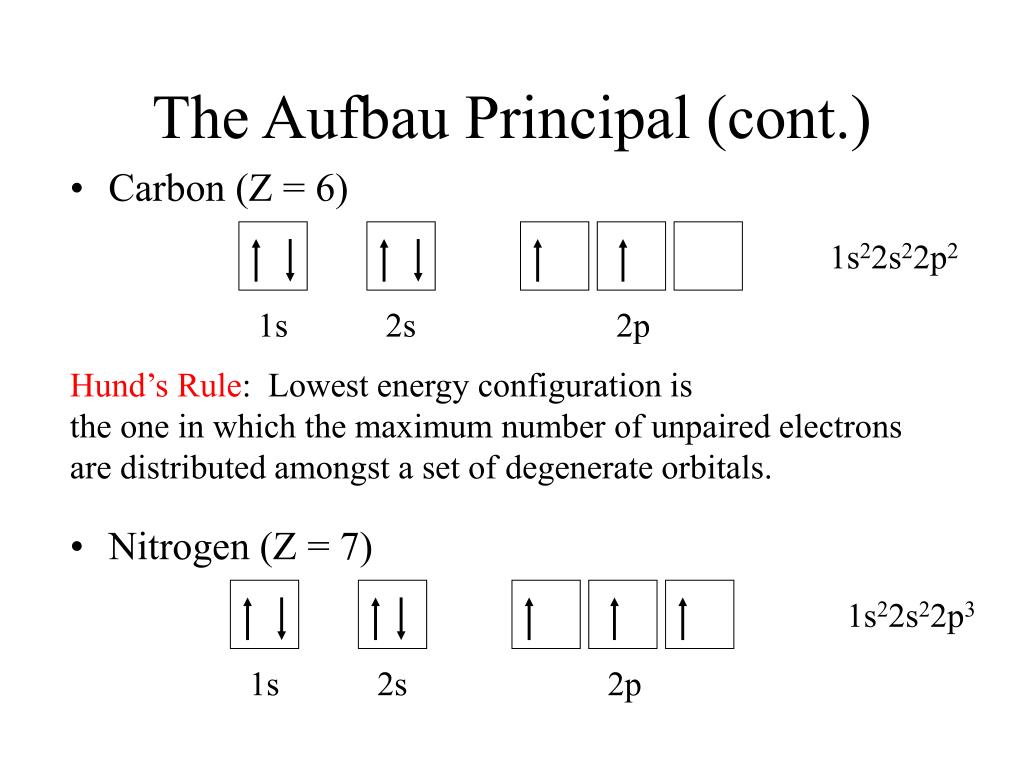
(a)This diagram represents the correct filling of electrons for the nitrogen atom.Pauli Exclusion Principle.
598K subscribers. According to this principle, electrons first fill up the lower energy subshells before progressively .This principle aids in the electronic configuration of atoms as well as the placement of electrons in orbitals.The Aufbau principle is a method of explaining the arrangements of electrons within atoms of different chemical elements.