Beer's law concentration curve

Chem 142 lab 4
64 x 10-6 M = .
Beer
com
Beer’s law
25 at 542 nm? The constant ε is specific to each solute at each wavelength and the .
2509 Spectral Analysis of Cu2+
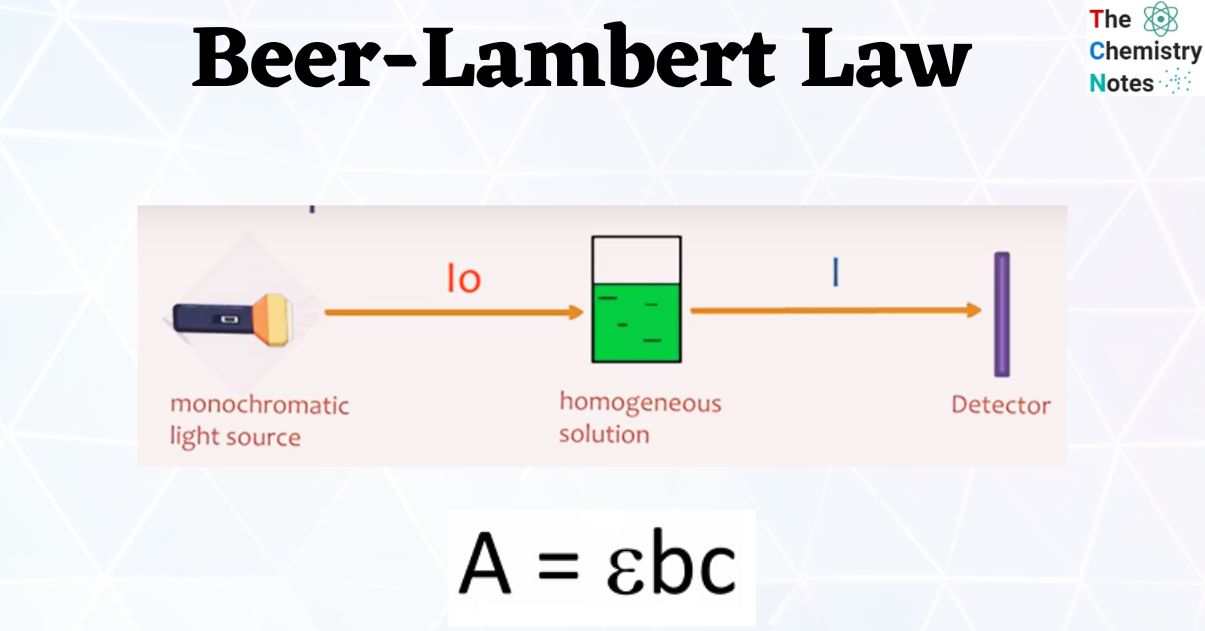
Beer's Law Equation and Example
University University of Washington . Thus, the slope of . Light source Sample Photodetector Figure 7.
In a similar way, . When the calibration curve is not a straight-line, the method’s sensitivity is a .Check me out: http://www.Beer's Law forms the basis for the analytical use of spectroscopy to determine concentrations. What you do is make up a number of solutions of the compound you are investigating - each of accurately known . Doing it this way you don't have to rely on a value of molar absorptivity, the reliability of the Beer-Lambert Law, or even know the dimensions of the cell containing the solution.In this lab you will construct a standard Beer's Law plot and use this curve to determine the concentration of FD&C Blue #1 in sports drinks.

142 Lab 4 Report: Calibration Curves and Beer's Law (take-home) Course.Step 2: Prepare a Calibration Curve. The graph should plot concentration (independent variable) on the x . Transmittance and Absorbance. An example of a Beer’s Law plot (concentration .
How to Calculate Concentration from Absorbance in Excel
Ideally, absorbance defined in this way is proportional to concentration, which simplifies analytical calibration.
Determining the Concentration of a Solution: Beer's Law
By using Beer’s law, we will calculate the concentration of the sample.
Manquant :
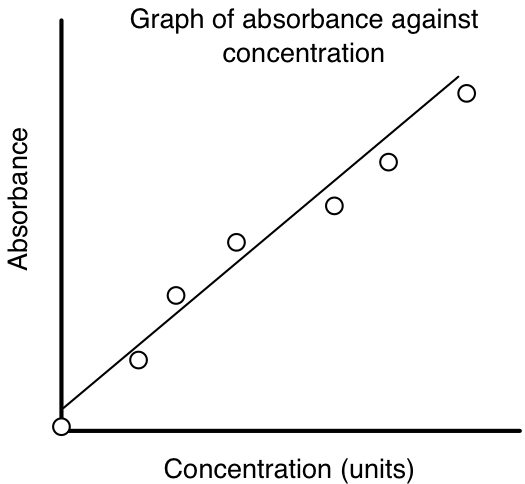
concentration curve It's important to understand that the deviations from the Beer-Lambert Law discussed here are not actually failures of this law but rather apparent deviations caused by failures of . This involves measuring the absorbance of samples with known concentrations and plotting the results on a graph.Graphing a calibration curve using solutions of known concentration lets you find an unknown concentration. Second Order Reaction.Beer -Lambert Law : Concentration, Path-length and Absorbance Calculator.Experiment 612: Beer’s Law . Determine the concentration of copper(II) ions in solution using spectrophotometric analysis. So let's write the equation of a line, Keeping in mind that . Values of alpha are tabulated for many common molecules, but the trouble is that alpha varies as a . As light passes through a sample, its power decreases as some of it is absorbed. To prepare a standard (calibration) curve for a spectroscopy experiment, start by preparing _____ with .This is an example of Beer's Law.2 , the slope of the line gives the value of \(b\varepsilon\) (where \(b\) is path length) This is the most desirable situation because the method’s sensitivity remains constant throughout the analyte’s concentration range. Many substances absorb UV (ultraviolet) or visible light. Next, you’ll want to prepare a calibration curve.Simple: 1) Find the most absorbed wavelength in your sample using a spectrometer. 2) Accurately measure the colour of multiple concentrations of your sample. The graph only applies to dilute solutions. The equation for Beer's law is: A = εmCl.by using a calibration curve of the absorbance over the concentration.Beer's Law Tutorial
The solution to this problem can be set up using the equation shown below, which simply says that the ratio of the concentrations is proportional to .
2508 Beer’s Law
Manquant :
concentration curveSpectrophotometry and the Beer
Duke University.You'll ultimately relate the UV-Vis absorbance intensity to the concentration of the [Fe (NCS)] 2+ complex using Beer's law. This allows you to experimentally measure the . [ Content Standard Unifying Concepts - Change, constancy, and measurement] The concentration of an unknown . Thus, a new approach to the accurate calculation of deviation from Beer's law has been developed.
4, which establish the linear relationship between absorbance and concentration, are known as Beer’s law.Calibration curves allow instrument calibration by predicting the concentration of an analyte in a sample from the reading of the instrument.The Beer–Lambert law relates the absorption of light by a solution to the properties of the solution according to the following equation: A = εbc, where ε is the molar absorptivity of . Summary of Rate Laws. 1cm is the width of a cuvette.Beer’s Law describes the linear relationship between the absorbance of electromagnetic radiation (i.Create a calibration curve by plotting the absorbance at λ max for the standard solutions with respect to their [Fe(NCS)] 2+ concentrations.This relationship is known as Beer’s law and is given by the equation: A= εbC . This relationship is used extensively in a variety of chemical analyses, from simple test kits that produce a color in the presence of certain analytes to high-end liquid chromatography instrumentation.bration curves based on Beer’s law are common in quantitative analyses. calculate the concentration of the sample. (A=absorbance, εm = molar extinction coefficient, C = concentration, l=path length of 1 cm) You should have a data set which was used to create a standard curve.Beer’s Law Example Calculation: A sample having a maximum absorbance value of 275nm. This page takes a brief look at the Beer-Lambert . “The thicker the glass, the darker the brew, the less the light that passes through.A non-invasive alternative of blood glucose monitoring is introduced in [6], based on the detection of the optical density of the solution samples by means of a spectrophotometer, and then converting it into the corresponding glucose concentration by using the Beer Lambert Law, with the help of a concentration. As indicated by equation \(\PageIndex{3}\), a plot of the absorbance at a given wavelength for a particular species versus the concentration of the species yields a straight line with a slope equal to \( {\epsilon}b \) and an intercept of zero .This video explains how the equation for Beer's Law can be used with a calibration curve to determine the molar absorptivity constant. Academic year: 2021/2022.” Make colorful . If there are no interactions between the components, then the individual absorbances, Ai, are additive. Luckily, the relationship is linear, and all we need to figure out is the slope from the standard curve. A plot of A vs C is called the analytical curve or the calibration curve. This is actual for the precise definition of the concentration ranges to reduce systematic errors on spectroscopic measurements when the strict position of the threshold point on the absorbance curves is unknown or is .Overview
Beer's Law
This attenuation of radiation is .When a calibration curve is a straight-line, as it is in Figure 2.Critiques : 47You will be applying Beer's law to calculate the concentration.In the context of lack of knowledge of the (constant) value of ɛ L, the following misuse of the Beer–Lambert Law is usually committed: in order to construct the calibration curve to predict the concentration of . As derived by Max Planck in 1903 from dispersion theory, Beer’s law has a fundamental limitation.The amount of light absorbed by a solution is related to the analyte concentration by the Beer–Lambert law, which is expressed as follows: A = εbc, where ε is the molar .Equation \ref{BL2} and Equation \ref{BL3}, which establish the linear relationship between absorbance and concentration, are known as Beer’s law. We recommend using the latest version of Chrome, Firefox, Safari, or Edge.Beer’s Law states that absorbance of a sample ( Abs) depends on the molar concentration ( c ), light path length in centimeters ( l ), and molar extinction coefficient ( ε) for the dissolved substance at the specified wavelength ( λ) [1]. The graph should have concentration on the x-axis and absorbance on the y-axis.4 Beer’s Law and Multicomponent Samples We can extend Beer’s law to a sample that contains several absorbing com-ponents.CHEM 142 Experiment #4: Calibration Curves and an Application of Beer's Law Goals of this lab: • Apply the use of a calibration curve to finding the concentration of an unknown • Apply the mechanics of dimensional analysis to calculate the mass of iron in a sample based on concentration of an iron-containing solution • Develop lab skills in operating digital .Beer's Law Beer's Law explains the relationship between cordial concentration and light .
Beer's Law Lab
Schematic diagram of a . Uploaded by: Anonymous Student.Concentration dependence of the absorbance at the oscillator position (1700 cm −1, black curve) compared to the normalized integrated absorbance (green line) and .The Beer-Lambert law relates the attenuation of light to the properties of the material through which the light is traveling.In the context of lack of knowledge of the (constant) value of ɛ L, the following misuse of the Beer–Lambert Law is usually committed: in order to construct the calibration curve to predict the concentration of an unknown solution from its known absorbance, a set of standard concentrations within the range of the measuring instrument are prepared, and ., light) and concentration.
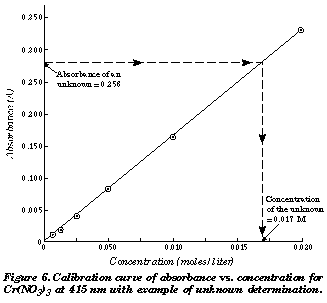
A = Absorbance ; ε = Molar absorption coefficient , Molar absorptivity or Molar extinction .Using this calibration curve the concentration of an unknown Rhodamine B solution can be determined by measuring its absorbance which is the main utility of the Beer-Lambert Law. For a two-component mixture of This curve is constructed as the . What is the concentration (in M) of a sample of the unknown dye with an absorbance of 0. A spectrophotometer value detected A=0.Temps de Lecture Estimé: 8 min This document has been uploaded by a student, just like you, who decided to . It is often referred to as Beer's law. Although very common, Beer’s Law is not the only source of application of calibration curves in different fields.
When a substance absorbs certain frequencies (colors) of light, some of them are transmitted, and the solution appears . Note: Beer's law is expressed by a linear function, which relates absorbance to concentration. Absorbance Spectra.


Calibration curves .This relationship is known as Beer’s law and is given by the equation: A= εbC (equation 4) where A is the absorbance of the solution, ε is the molar absorptivity of the solute, b is the .
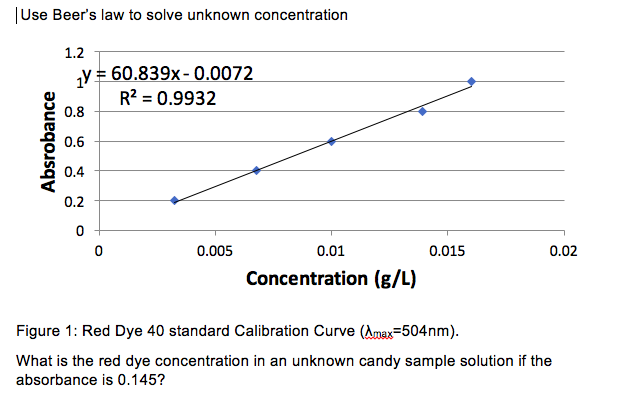
From the Beer-Lambert law (where c is concentration) 0.The PhET website does not support your browser.
Calculating concentration using the Beer
where A is the absorbance of the solution, ε is the molar absorptivity of the solute, b is the path length of light passing through the sample of solution, and C is the concentration of the solution in molarity (moles/L).If you solve Beer's Law for concentration, you get: c = A / (L* alpha) So, you could determine the concentration c simply by measuring the absorbance A and dividing it by the product of the path length L and absorptivity alpha. Students shared 126 documents in this course. Kinetics Decay Plots. The concentration dependence of absorbance can deviate from linearity, even in the .You will graph this direct relationship between concentration and absorbance and use the straight line to find an unknown concentration of the same substance.