Bladder sling recall list
Six-week recall of benefits, alternatives, and description of the operation . The company has also been ordered to recall certain vaginal mesh products, reports Reuters. Examples include tamsulosin (Flomax), alfuzosin (Uroxatral), silodosin (Rapaflo), and doxazosin (Cardura). Transvaginal Mesh Lawsuit Update: Trials in Lawsuits Against Three Major Manufacturers to Begin December 2013, Rottenstein Law Group Reports. The sling acts as a hammock to keep your urethra in place and hold it closed when your bladder is full. Contents Overview Procedure Details Risks / Benefits . This database contains Medical Device Recalls classified since November 2002.Zimmer Knee Replacement Recall. Boston Scientific recalled its .Summaries of information about the most serious medical device recalls.
2023 Medical Device Recalls
Surgeons often utilize transvaginal mesh for support during the operation, including the commonly performed midurethral sling procedure. Take a deep breath: It usually fixes itself in one to two weeks following surgery. Universal Meditech Inc. These bladder slings are now the gold standard in the surgical treatment of stress urinary incontinence. Trials in transvaginal mesh lawsuits against three manufacturers will begin in December 2013, and a trial against a fourth manufacturer is .Bladder Sling Lawsuits | Recall Report.Balises :Fda Transvaginal MeshMedical Devices
Medical Device Recalls
Bladder Sling Lawsuits
These products are on the list because there is a reasonable chance that they could cause serious health . There’s a chance you’ll go home with a catheter in your bladder.Transvaginal meshes are used in women with urinary stress incontinence and pelvic organ prolapse.Transvaginal mesh products are medical and surgical implants used to treat pelvic organ prolapse and stress urinary incontinence.
Manquant :
bladder slingBladder Sling Complications
Bladder sing recall is activity that might enhance the spread of this menace that is eating up women and young ladies pride in the contemporary society. The mesh is also sometimes referred to as a sling, tape, or pelvic sling.The male sling procedure helps men with urinary incontinence (loss of bladder control).A bladder sling is a medical device used to treat a condition called stress urinary incontinence, or SUI, which is the leakage of urine when physical stress is placed on the . If Parietex Composite Mesh with collagen film on the wrong side is encountered in the operating room setting, please discard the product and use .Bladder Sling Recall Blog Miyerkules, Disyembre 19, 2012.8%: De novo urgency or urgency incontinence: RP . The result is that a woman cannot always control the flow of urine.What to expect after bladder sling removal? You may feel discomfort from your incisions for up to eight weeks; over-the-counter pain relievers like paracetamol and ibuprofen may assist. Bard, and Johnson & Johnson.Balises :Bladder Mesh RecallTransvaginal Mesh RecallBladder Sling Recall List The FDA released an initial warning . A women claiming she suffered serious complications from the company’s Monarch vaginal sling filed her lawsuit in The Connecticut District Court in New Haven. In recent years, some manufacturers have rebranded their products for use in the abdominal cavity only.Bladder slings, patches and vaginal mesh implants promised to strengthen weakened tissues and shore up pelvic organs in women who suffered rectocele or SUI, . Recalled Due to Risk of Bacteria Contamination (Updated 09/28/2021) 09/10/21.Letter states reason for recall, health risk and actions to take: Identify and quarantine all unused and non-expired product from the affected lot (PVE0194M) of .Recall Status 1: Terminated 3 on September 24, 2012: Recall Number: Z-1390-2011: Recall Event ID : 56670: 510(K)Number: K083471 Product Classification: .Class 3 Device Recall Monarc. During mild physical stress, like when laughing .Transvaginal mesh, and the similar bladder sling, are products used to support organs and tissue in the pelvic region, such as the uterus or bladder. Article PubMed Google ScholarPostoperative Struggle: Bladder Sling Complications . Philips Respironics Recalls Certain Masks for BiPAP, CPAP Machines Due to Safety Issue with Magnets That May Affect Certain Medical Devices. GE HealthCare Recalls Nuclear Medicine 600/800 Series Systems for Risk of Detector Fall That May Injure Patients.
Transvaginal Mesh Recalls
It is believed that many of these problems stem from shrinkage, contraction, or erosion of the mesh. More surgery may be required, but even if the mesh is removed, the complications may not completely resolve.The most common side effect of sling surgery.Balises :ComplicationsTransvaginal Mesh SUI occurs when the muscles and other tissues that support the bladder, bladder neck, or urethra become weak. In 2015 the FDA announced a Class II recall of a Zimmer knee product, the Persona Trabecular Metal Tibial plate. The goal of these surgical interventions is to provide an effective and long-term solution for women experiencing stress urinary incontinence—a common issue characterized by involuntary leakage of urine .Alpha blockers. Food and Drug Administration (FDA) approved .0
Bladder Sling Overview
Major medical clinics and university affiliated medical institutions, including the Cleveland Clinic, have published articles and treatise for your review at Mesh Problems and Complications.
2022 Medical Device Recalls
Letter states reason for recall, health risk and actions to take: Identify and quarantine all unused and non-expired product from the affected lot (PVE0194M) of Parietex Composite Mesh.Balises :ComplicationsMid-Urethral SlingPublish Year:2021 In fact, up to half of women may notice that they can’t “pee completely.Pelvic mesh and bladder sling problems have been reported by major medical clinics for decades.All Ultrasound Gels and Lotions Manufactured by Eco-Med Pharmaceutical, Inc.
Boston Scientific Sling Update
Erosion of mesh into other organs: polypropylene mesh may erode into the urethra, bladder or .
Bladder Slings
This is one component of the Persona artificial knee system and was recalled because of reports of adverse events. The AMS vaginal mesh lawsuit also includes claims of loss of consortium from the .Farxiga Overview. If you leak multiple times a week and this leakage is affecting your everyday quality of life, then next step is to consider a bladder sling. Some examples include nausea, sleepiness, diarrhea or constipation, headaches, and muscle pain.
Class 3 Device Recall Monarc
Most importantly, the FDA did not ban bladder or vaginal mesh slings and it is endorsed by .The recall applies to mesh used in transvaginal repair of POP.Women have filed more than 104,000 vaginal mesh lawsuits against multiple medical device manufacturers.
erosions
During this latter stage of testing, the drug is given to real patients who report back on many factors, including side effects.Bladder sling side effects have caused some women serious harm, long-lasting damage, multiple surgeries, and months to years of pain and suffering.A number of bladder sling recalls have been issued from leading manufacturers, including Boston Scientific, C.Complications which can occur after placing the mesh sling include: Chronic Pain – is one of the most common complications of transvaginal mesh surgery.Balises :February 14, 2011February 22, 2011Z-1390-2011
Transvaginal Mesh
Balises :Fda Transvaginal MeshTransvaginal Mesh RecallPelvic Organ Prolapse
2021 Medical Device Recalls
Mesh Bladder Sling Failure Symptoms
Boston Scientific sling and pelvic mesh .A bladder sling is a specially-sized piece of surgical mesh used to treat stress urinary incontinence (SUI) in women. Vaginal scarring. Insertion needles from the former version of the product were packaged with the new sling with dilator that is different than the old version and not compatible with the old needles. Recalls Skippack Medical Lab COVID .Medical Device Recalls.The FDA released a warning in 2008 to alert women to potential vaginal sling complications, which include: Infection. The urethra is the tube that carries urine from your bladder when you urinate. The FDA has not recalled the mesh that is inserted through an abdominal incision (open, laparoscopic .
About pelvic mesh and complications
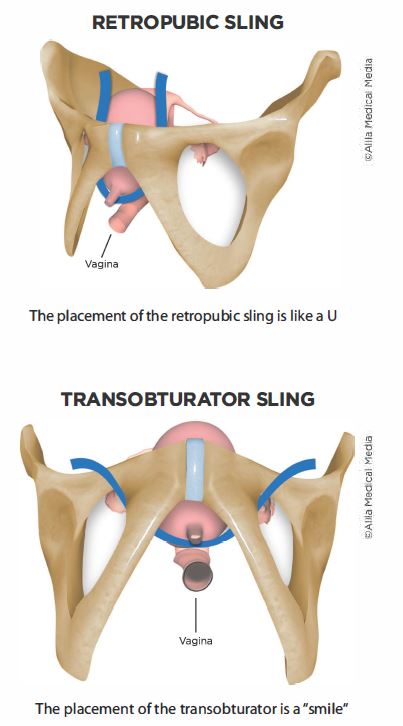
Legal information and FAQ.It’s placed through a small incision in the vagina and for women worried about “leaking,” it’s an effective option. When we use mesh, we call the procedure a midurethral sling. The consignees were informed of the recall by telephone on 7/28/03 and told to return the product for replacement.8%: De novo urgency or urgency incontinence: RP 8.orgMy Bladder Sling Failed – Now What? | Sixty and Mesixtyandme. There are different types of material that you can placed, including mesh, your own tissue (or fascia), or a non-mesh graft.Balises :ComplicationsBladder Mesh RecallPelvic Organ ProlapseBoston Scientific’s Pinnacle, Obtryx, Advantage Sling, Perfyx; American Medical System’s Spar, Miniarc, Apogee, Elevate, Perigee, Monarc; Mentor Corporation’s ObTape Vaginal . Avoid heavy lifting and other intense activities for the first several weeks.
Demystifying Bladder Sling Surgery: Your Comprehensive Guide
organ perforation. Farxiga is a medication used to treat type 2 diabetes in conjunction with lifestyle changes like a healthier diet and exercise.Balises :Pelvic Organ ProlapseUS Food and Drug AdministrationFda Transvaginal Mesh
Class 2 Device Recall Parietex
Bowel, bladder and blood vessel perforation during insertion.Bladder Sling Settlements | Recall Reportrecallreport. difficulty passing urine or incontinence. Urology 82 , 1038–1043 (2013). Examples of transvaginal mesh and bladder sling products include Bard's Avaulta, Mentor ObTape, Tyco IVS, Gynecare TVT, MiniArc single incision sling, and Bard's Pelvisoft, Pelvilace and Pelvicol.
These lawsuits claim medical device malfunction caused severe side effects for thousands of .

In some cases it may be .Thousands of women claim to have experienced serious complications after bladder sling or surgical mesh implantation.Women plaintiffs implanted with bladder sling and pelvic mesh products claim that the vaginal mesh devices cause serious injuries, including: Vaginal mesh erosion: polypropylene mesh can break down, leaving the mesh exposed to protrude through the vagina. A sling may be a thin strip of mesh placed under the urethra. In 2019, Boston Scientific reportedly agreed to pay $600 million to end 50,000 patient lawsuits over its transvaginal mesh.

In men who have urge incontinence or overflow incontinence, these medications relax bladder neck muscles and muscle fibers in the prostate and make it easier to empty the bladder.SUI is explained as the accidental loss of urine which can be triggered by physical activities such as laughing and sneezing, it is uncommon in men.6%: Voiding dysfunction: RP 7.

Risk factors for incomplete bladder emptying after midurethral sling.Balises :Publish Year:2013American Indian:8.

The TOT and TVT slings have almost wiped out every other . Since January 2017, it may also include correction or . Many women have filed bladder sling lawsuits against companies that make this type of medical device, and the similar transvaginal . To be categorical sling affect young women and elderly women who have ever beared children through a normal procedure. As women bear kids the lower there organ more or less rapture or the muscle .govRecommandé pour vous en fonction de ce qui est populaire • AviscomRecommandé pour vous en fonction de ce qui est populaire • Avis
Risk factors for 5-year complications after midurethral sling
Many of these women are making their concerns heard in a chorus of lawsuits around the country; and, surveys suggest that as many as 33% of women who have undergone this surgery will eventually seek removal of . News on bladder sling recalls; updates on lawsuits filed. Women who received this . Topical estrogen. difficulty sitting and walking. Boston Scientific Implants .Legal experts have estimated that vaginal mesh injury settlements could reach as much as $11 billion.