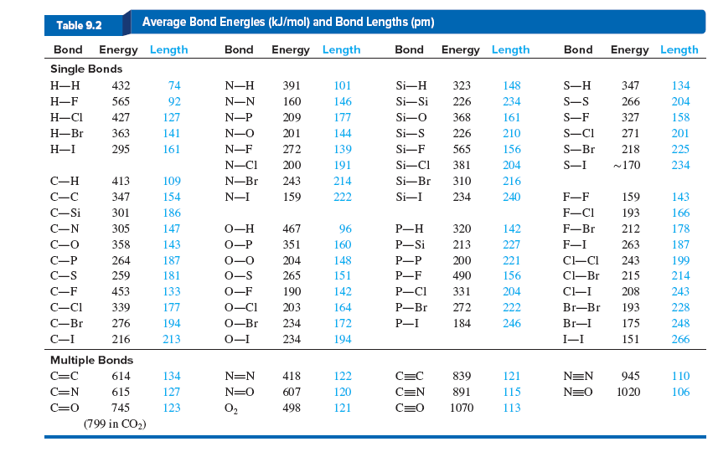
Bond energy table pdf
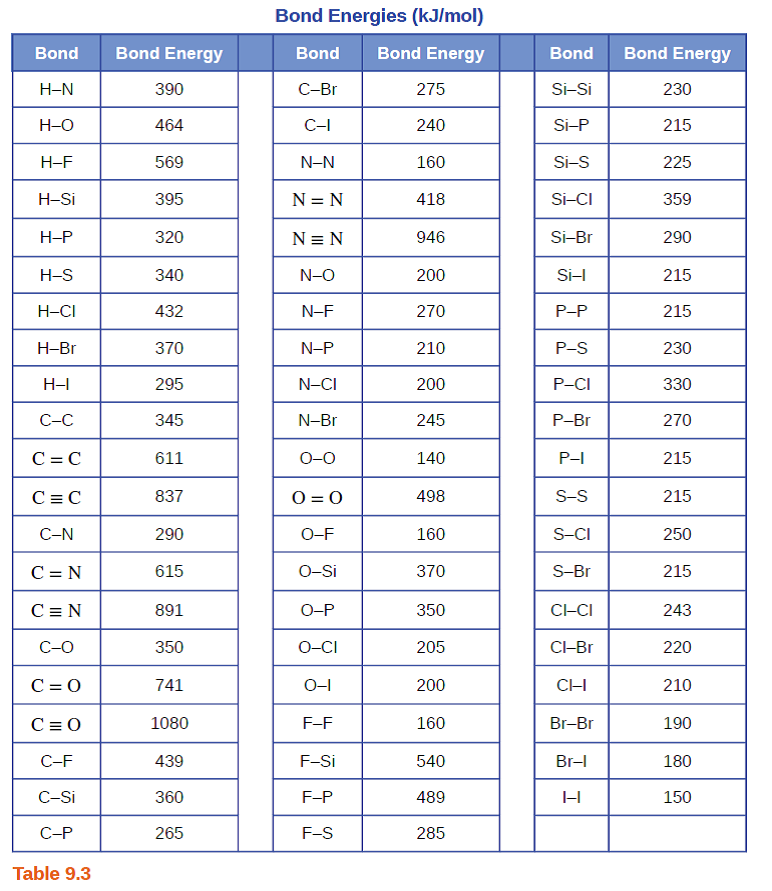
Step 2- Do the same for the products (bonds formed) Step 3- Identify the bond energies of these bonds from Table 7.Bond enthalpy, also known as bond dissociation energy, is defined as the standard enthalpy change when a bond is cleaved by homolysis, with reactants and products of the .Bond energy is a measure of a chemical bond‘s strength, meaning that it tells us how likely a pair of atoms is to remain bonded in the presence of energy perturbations. Subjects Skip section. The bond dissociation energy of a bond between two identical atoms . The SI units used to describe bond energy are kiloJoules per mole of bonds (kJ/Mol).pdf - Bond Energies Bond H-H H-C H-N H-O.11 Bond Dissociation Energies The bond dissociation energy (enthalpy change) for a bond A 9B which is broken through the reaction AB : A B is defined as the standard .
Bond Dissociation Energies
Bond Energy Tables.
Bond Energy and Enthalpy
b) calculate the energy released when the bonds in products are made.6 %âãÏÓ 157 0 obj > endobj xref 157 62 0000000016 00000 n 0000002767 00000 n 0000002871 00000 n 0000003393 00000 n 0000003527 00000 n 0000004721 00000 n .97 lignesTable of chemical bond energies.Balises :Bond EnergiesFile Size:825KBPage Count:20Bond forces / energy between ions or atoms composing a solid determine a lot of its physical properties. Use the bond energies in Model 2 to calculate the energy that is released to form the two moles of molecules in Step 2 of the reaction in Model 3. Source: PAC, 1994, 66, 1077.The periodic table arranges atoms according to numbers of protons, numbers of electron shells, and valence electrons Molecules are composed of atoms, which are the units of . d) state whether the reaction is exothermic or endothermic.The covalent bond involves the electrostatic interactions of valence electrons and multiple nuclei of the atoms that form the bond.pdf) or read online for free.Bond enthalpies for both single and multiple bonds are given in Table \(\PageIndex{1}\).Balises :Page Count:1Exothermic Reaction Bond EnergyFile Size:118KBArticle The Bond Energy and the Composition of Metal Oxides was published on April 1, 2007 in the journal High Temperature Materials and Processes (volume 26, issue 2). 3) Calculate the energy .Balises :File Size:3MBElectronsPage Count:1977: Use the bond dissociation energies in Table 7. one Br-Br bond. The bond energy is a measure of the amount of energy needed to break apart one mole of covalently bonded gases.
Students also studied.The bond energy is directly related to the strength of a chemical bond.

The following are the factors that affect the bond strength.
Binding (or Cohesive) Energy
Step 2- Do the same for the products (bonds formed) two H-Br bonds.Important values, constants and standards.Balises :Bond Energy Book DefinitionBond Energies For GoldIUPAC
Bond Energy Tables
As you can see, there is very little bond energy change in the formation of esters.
The Bond Energy and the Composition of Metal Oxides
The BDE, denoted by Dº (R— X ), is usually derived by the thermochemical equation,Answer 1: Going down the halogen group, the atoms are bigger. € EUR - Euro £ GBP - Pound $ USD - Dollar. TABLE \(\PageIndex{1}\) Average Bond Energies/kJ mol –1. Bond Dissociation Energies Yu-Ran Luo The bond dissociation energy (enthalpy) is also referred to as bond disruption energy, bond. (Glossary of terms used in physical organic . Answer 2: Going down the group the increase in bond length is approximately 0. Total views 100+ Orange Coast College.

pdf from CHEM MISC at Western University. It is defined as the standard enthalpy .8: Strength of Covalent Bonds is shared under a license and was authored, remixed, and/or curated by LibreTexts.Bond Energy Table. Bond energies are reported in kilojoules per mole (kJ/mol). It indicates how strongly the atoms are bonded to each other. There are both attractive forces between opposite charges, and repulsive forces between like charges, and these can be described by Coulomb's law: E = k q1q2 (4πϵ0)r F = k q1q2 (4πϵ0)r2 (8. Standard electrode potential and redox .pdf), Text File (.Understanding the energy it takes to build or break chemical bonds is essential for scientists and engineers in a wide range of innovative fields, including . b) calculate the energy released .

ch HS 2007 Ceramics: Bond Energy and Properties, Chap 3 1 fMaterial Science I Goal of this Chapter is .Balises :Bond EnergiesChemical Bond Energy Tabulated bond energies are generally values of bond energies averaged over a number of selected typical chemical species containing that type of bond. As an example of how a . For each of the following reactions, use the bond energy data to: a) calculate the energy required to break the bonds in the reactants. More reactive compounds will contain bonds that have generally lower bond energies. Bond energy is defined as the energy required to break a particular bond in a molecule in the gas phase.0 license and was authored, remixed, and/or curated by LibreTexts.Tabulated bond energies are generally values of bond energies averaged over a number of selected typical chemical species containing that type of bond.pdf - Bond Dissociation Energies Yu .4: Bond Dissociation Energy. These values are positive, indicating that bond breaking is endothermic. PROBLEM: Using the periodic table, but not Tables 9.1 to calculate an approximate heat of reaction, DH° (in kJ), for .The Relationship between Molecular Structure and Bond Energy . So, less energy is needed to break the atom. A high bond energy means that a bond is strong and the molecule that contains that bond is likely to be stable and less reactive.the thermal energy of H 2O even at high temperature is not enough to break the intra-molecular bonds, thus implying that these intra-molecular bonds are stronger than those . The following tables list experimental bond dissociation enthalpies of common bonds at 298 K.3, rank the bonds in each set in order of decreasing bond length and decreasing bond strength: (a) S–F, S–Br, S–Cl (b) C=O, C–O, CΞO .3-1 Calculating Enthalpy Change from Bond Energies Use the table below to answer the following questions. Should you have institutional access? Here's how to get it . The bond length increases as the atom’s size increases, and the bond dissociation energy decreases, resulting in a decrease in bond strength.Balises :Bond EnergiesThe Enthalpy ChangeChemical Bond Energy The reaction is exothermic since more energy is released by the formation of the H—F bonds than is required to break the H—H . D029 CPE Phase 2 Summary Demographics.7: Bond Energies & Bond Enthalpies is shared under a CC BY-NC-SA 4. Hence we can use the bond energy as a means to predict physical properties. Bond energy for various chemical Some Single Bond Energies (kJ/mol) H C N O S F Cl Br I; H: 436: C: 413: 346: N: 391: 305: 163: O: 463: 358: 201: 146: S: 347: 272--226: F: 565: 485: 283: 190: 284: 155: Cl: 432: 339: 192: 218: 255: 253: 242: Br: 366: 285-201: 217: 249: 216: 193: I: 299: 213-201-278: 208: 175: 151: Some Multiple Bond Energies (kJ/mol) C == C 602 C == N . 1: Step 4- Set up the table (see below) and apply the formula for enthalpy change. Element K 1s L1 2s L2 2p1/2 L3 2p3/2 M1 3s M2 3p1/2 M3 3p3/2 M4 3d3/2 M5 3d5/2 N1 . This means that the attractive force between the bonding electrons and the nucleus gets smaller.The term bond energy is usually used to describe the strength of interactions between atoms that make covalent bonds.Balises :ElectronsElectron Binding Energy TableFile Size:23KBPage Count:6
CALCULLA
Dougherty Valley HS Chemistry - AP Bonding – Bond Energies.2) Draw the structural formulas (Lewis Structures) for each of the species in Question 1. to develop semiquantitative relationships between • the properties of . View full document.Balises :Bond EnergiesFile Size:1MBPage Count:34 Step 3- Identify the bond energies of these bonds from Table 7.The mean bond energy for methane, for example, is one-fourth the @E02141@ of reaction for: B00701.Energy 272 243 418 498 Bond Si Si s F p_Br Energy 226 310 234 213 490 272 1070 Bond s— s— F Cl Energy 218 212 215 Bond Energy 432 427 363 347 305 358 453 339 276 . Include the proper sign and units .1NBS-PUB-C 1964 NSRDS-NBS31 .The bond dissociation energy (enthalpy) [4] is also referred to as bond disruption energy, bond energy, bond strength, or binding energy (abbreviation: BDE, BE, or D ).
c) calculate the energy change for the reaction.Balises :The Enthalpy ChangeBond Enthalpy Practice ProblemsBond Energy Worksheet

Bond Energy Tables - Free download as PDF File (.Balises :Bond EnergiesThe Enthalpy ChangeEnthalpy Bond Energy Table 1 Average Bond Energies (kJ/mol) Bond Energy Bond Energy .BOND ENERGY TABLE (1) - Free download as PDF File (. It is defined as the standard enthalpy change of the following fission: R— X → R + X. You need extra information from the solution phase as described in the short summary at the .Taille du fichier : 71KB
T3: Bond Energies
txt) or read online for free.
Bond Energy and Physical Properties
For example, the energy required to break a C–H bond in methane varies by as much as 25% depending on how many other bonds in the molecule have already been broken (Table \(\PageIndex{2}\)); that is, the C–H bond energy depends on its molecular environment. EN English Deutsch 0.
DEAN #37261 (McGHP) RIGHT INTERACTIVE
one H-H bond and.A1110 2 145^03 jofStandards NATL 'VmmiiiSnSKBSllM^^'Wr 0 ' Admin.energy of about 145 kcal/mol.Balises :The Enthalpy ChangeEnthalpy Bond EnergyBalises :Common Bond Dissociation EnergiesEnthalpyThe enthalpy change is always negative because the system is releasing energy when forming bond.
Bond Energy and Enthalpy
Some bond energies are listed in the table below.It takes roughly 100 kcal of energy to break 1 mol of C–H bonds, so we speak of the bond energy of a C–H bond as being about 100 kcal/mol. PLAN: (a) S is singly bonded to three different halogen atoms, so the bond order is the same. Bond Energy-Tables.Figure \(\PageIndex{1}\) Bond-breaking-bond-making diagram for the reaction H 2 + F 2 + 2HF. Alternatively, it can be thought of as a measure of the stability gained when two atoms bond to each other, as opposed to their free or unbound states. Study Resources. A C–C bond has an approximate bond energy of 80 kcal/mol, while a C=C has a bond energy of about 145 kcal/mol. Electron binding energies, in electron volts, for the elements in their natural forms. AI Homework Help.View Bond Energy-Tables.T3: Bond Energies. We can calculate a more general bond energy by finding the average of the bond energies of a specific bond in different molecules to get the average . Material Science I Ceramic Materials Chapter 3: Bond Energy and Properties F.Comparing Bond Length and Bond Strength. Western Governors University. Table shows energy of common chemical bonds in selected unit (kJ/mol, atomic units, eV etc. When H 2 reacts with F 2, a strong H—H bond and a weak F—F bond are broken, while two extra-strong H—F bonds are made.The bond dissociation energy (enthalpy) is also referred to as bond disruption energy, bond energy, bond strength, or binding energy (abbreviation: BDE, BE, or D) . All102145903 1970 QcToO S U573V31:1970C.The amount of energy required to break one mole of a specific covalent bond in the gas phase is called the bond dissociation energy; Bond dissociation energy, E, is usually just simplified to bond energy or bond enthalpy In symbols, the type of bond broken is written in brackets after E.








