Bsi audit medical device
I’m delighted to be given this exciting opportunity at BSI.Design and develop medical devices to international quality standards, ensure smooth submission, meet ISO 13485 standards, satisfy customers and keep ahead of all regulatory developments with our medical devices training courses. Quality System Audit Fees: Medical Device QMS Audit (Excul svi e of travel tmi e and expenses) €245 per hour . Verify a Certificate. 1 Introduction.Medical Device Single Audit Program (MDSAP) > .
Single audit program for medical device QMS audits taking off
BSI runs online, public or in-house training courses.We are a global organization, trusted and recognized around the world. It could involve a reduction in incidents, improved processes or increases in performance. We offer a wide range of free and live webinars hosted by BSI Technical Specialists addressing key topics that affect your business including legislation, risk, and regulatory changes. BSI The Netherlands (2797) is a leading Notified Body; we review medical devices to ensure that they conform to the requirements of the European Directives and Regulations.BSI's approach to excellence. Select an upcoming webinar below to register your interest.In this section: Medical Device Single Audit Program (MDSAP) Medical Device Single Audit Program (MDSAP) MDSAP Documents; Home . Popular searches. Alternatively you can watch all of our on-demand . Die Benannte Stelle - BSI Niederlande (2797) prüft Medizinprodukte, um ihre Konformität mit den europäischen Richtlinien und Verordnungen zu gewährleisten. Course Duration. Our Hybrid Audit programme has been developed using immersive . Search BSI; Verify a Certificate; Search BSI. The Medical Device Single Audit Program was developed to conduct regulatory audits of quality management systems (QMS) of manufacturers of medical devices. Medical device live and on demand webinars. (BSI, une société constituée en vertu de la Charte Royale) exerce son activité en tant qu'organisme national de normalisation (NSB, National Standards Body) au Royaume-Uni.BSI Medical Devices is an accredited Certification Body for ISO 13485 in several global markets and a recognized Auditing Organization for the Medical Device Single Audit . Find more courses. We offer a wide range of free and live webinars hosted by BSI Technical Specialists . We are: A designated EU Notified Body.
Medical Device Single Audit Program (MDSAP)
Call: +1 800 862 4977. For external use MDF4206 Revision No 1 (October 2021) - Page 2 For external use Conformity assessment activities and their fees .Manufacturers with sterile medical devices under IVDR/MDR/MDD/IVDD When implementing the regulations, the depth and range of conformity assessment activities for sterile devices require significant additional resources for manufacturers.ISO 13485 Auditor Qualifications. Candidates must have design, manufacturing or process knowledge in addition .Before placing a medical device on the European market, manufacturers need to produce technical documentation providing evidence of conformity with the relevant legislation. We review your medical devices and IVDs to assess conformity against the applicable European legislations.
BSI UK (0086) is a UK Approved Body able to provide conformity assessments under the new .Un Organisme d'Audit reconnu dans le cadre du Medical Device Single Audit Program (MDSAP) Un Organisme de Certification reconnu dans de nombreux marchés . The two-day training is delivered either in person or live online in a classroom environment.

BSI is an Auditing Organization for MDSAP. The ability to identify opportunities for improvement is an important skill for an internal or lead auditor. Find out more >. BSI UK (0086) is a full-scope UK Approved Body assessing Medical Devices and IVDs and it is the only one reviewing all three . Level Auditing.The MDSAP allows a single audit conducted by a recognized Auditing Organization (AO) to cover the requirements of BS EN ISO 13485:2016, together with .Call: +1 800 862 4977.
BSI Training
Zertifizierung und Zulassung Medizinprodukte
This whitepaper seeks to review the history of 3D printing of medical devices, identify the key characteristics of successful exploitation, and to examine the scope for bioprinting processes to enhance medical devices, bearing in mind the lessons learnt from the more established 3D printing industry.Internal Auditor ISO 13485:2016 Training Course.Hybrid audits for medical devices > .
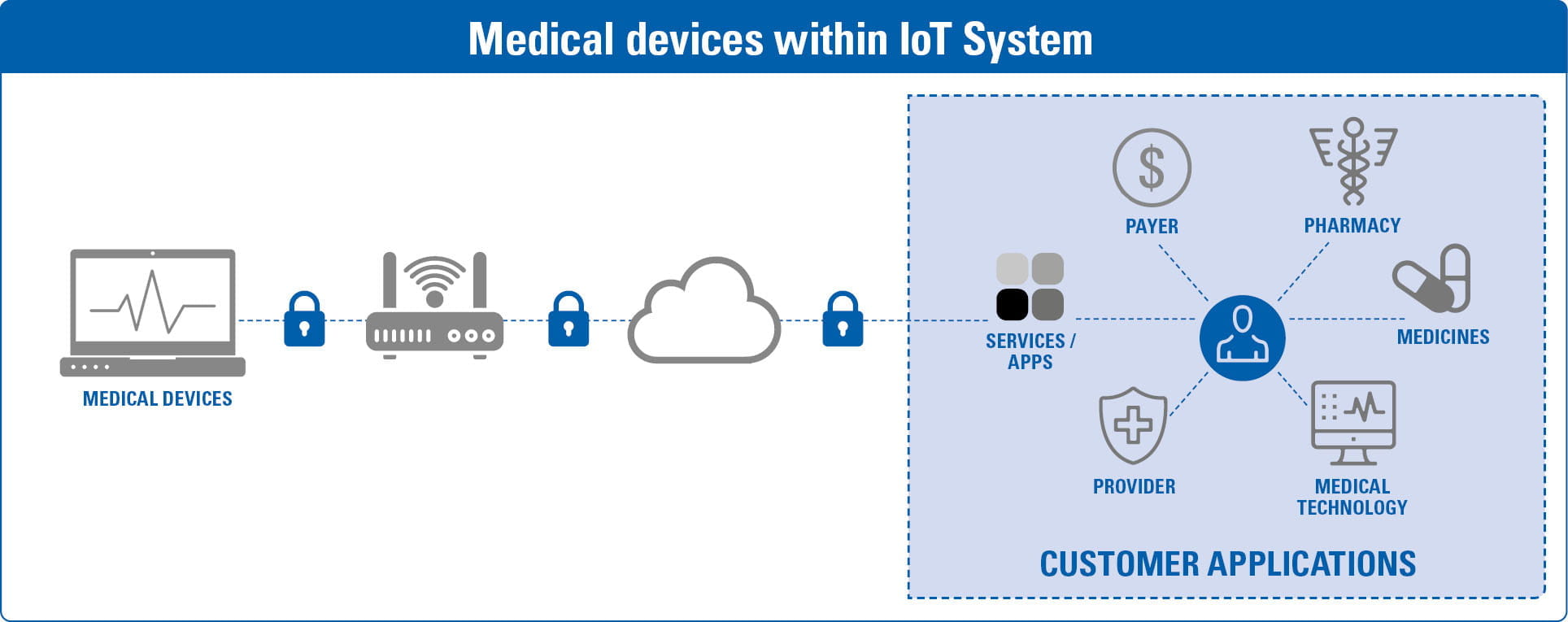
BSI, en collaboration avec les sociétés du groupe, propose également un large portefeuille de solutions commerciales autres que .
Quality Management System (QMS) Certification
Dispositifs Médicaux
Quality Management (ISO 9001) Information Security (ISO 27001) Environmental Management (ISO 14001) Occupational Health and Safety Management (ISO 45001) Carbon Footprint Verification (ISO 14064-1) Country selector.
Auditing and Verification Services for Protection
From supplier audits to internal audits, we can give you peace of mind that your supply chain requirements are met. 1 bsigroupcom Introduction Before placing a medical device on the European market, manufacturers need to produce technical documentation providing evidence of conformity with the relevant legislation. We have dynamic course owners around the world, allowing delivery of training in many local languages. Whether you want us to verify that a .The Medical Device Single Audit Program (MDSAP) allows a single audit of a medical device manufacturer’s Quality Management System (QMS), which satisfies the requirements of multiple regulatory jurisdictions. We are: A designated EU Notified Body; A UK Approved Body; An accredited ISO 13485 Certification Body; A recognized Auditing Organization under the Medical Device Single Audit Program (MDSAP) A recognized Certification Body in many global markets . A UK Approved Body. We are: A designated EU Notified Body; A UK Approved Body; An accredited ISO . Qualität beibehalten und .
Medical Devices
MD Services

Medical Devices. That's why we've developed auditor qualifications for ISO 13485 – medical devices quality management systems .) and reducing time to issue certification. Medical Devices Industry.BSI Medical Devices offers certification services to support your global market access goals.Medical Devices Regulation (MDR and IVDR) Effective 1 January 2022 . Stay up to date with latest news and announcements from BSI and the medical devices industry.Medical device single audit program MDSAP. MDSAP audits can be . Here at BSI, we also offer MDSAP in combination with CE, UKCA, ISO 13485 and ISO 9001 assessments.The Medical Device Regulation (MDR) is the legislation detailing the requirements that manufacturers must meet to place medical devices on the market in the European Union. View the latest news > Medical device training.
Hybrid audits for medical device manufacturers
TheMedical Device Single Audit Program - MDSAP - was developed by the .
Hybrid audits for medical device manufacturers
Part of the audit offering remains in-person and onsite, but a large proportion is now delivered remotely. The formation of a new, dedicated team will allow us to focus our attention on this challenging area of new EU .Our comprehensive review process combined with our world-leading experience as a notified body will ensure that your conformity assessment process is both efficient and . A BSI MDSAP audit can also be combined with assessment for CE and ISO 13485.Medical Devices Notified Body BSI (BSI-UK / BSI-NL) and medical device manufacturers both have an interest in speeding up the review of Technical Documentation (as part of initial approvals, substantial change approvals, renewal applications etc. The Medical Device Regulation (MDR) is the legislation detailing .
ISO 13485: Critical Subcontractors & Crucial Suppliers
The Role of a Medical Device Notified Body
The most common reasons for delays in Technical Documentation . BSI selects and recruits professionals to conduct ISO 13485 audits. Whether you are just starting out on your business improvement journey, or looking to enhance your current knowledge and capabilities, contact our expert team who will be able to give advice and guidance about options that will enable you to meet your goals.This time we’re excited to let you know that we’ve got an exceptional lineup of speakers from four Notified Bodies: Sharmila Gardner, Technical Documentation .Il Medical Device Single Audit Program (MDSAP) consente, ad un produttore di dispositivi medici, di ricevere un unico audit del Sistema di Gestione della Qualità (SGQ) che soddisfi i requisiti di più giurisdizioni normative.Note: In the context of the audit of medical device manufacturers, a critical supplier is a supplier of a product or service, the failure of which to meet specified requirements could . Technical documentation needs to be in compliance with the Medical Devices Directive (MDD) 93/42/EEC or the Active . As indicated in the EU MDD/MDR and UK MDR, standalone software which has a medical purpose is considered to be an active medical device.The European Commission’s guidance, MEDDEV 2. Designed by your partner in learning as an intensive course for medical device quality professionals, this ISO 13485 internal auditor training builds knowledge for an effective quality management system.The Medical Device Single Audit Program ( MDSAP) was conceived by the International Medical Device Regulators Forum (IMDRF) for the conduct of regulatory . To align with the overriding changes, the frequency of onsite microbiology audits will adjust .BSI has a very selective recruitment process for the professionals we hire to conduct ISO 13485 audits.BSI The Netherlands (2797) is a leading Notified Body achieving full-scope designation under MDR and IVDR.MDSAP is a way that medical device manufacturers can be audited once for compliance with the standard and regulatory requirements of up to five different medical device markets: Australia, Brazil, Canada, Japan and the United States.Medical Device Single Audit Program > . We are: A designated EU Notified Body; A UK Approved Body; An accredited ISO 13485 Certification Body; A . View all training courses > Events and .
Using the MDSAP to support EU regulatory requirements
Medical Device Single Audit Program (MDSAP) Fundamentals and Readiness Training Course.

As more and more manufacturers now have their MDR Quality Management System (QMS) certificates, it’s imperative for continued compliance that they are able to perform audits . Gli audit sono condotti da Organizzazioni di Audit (AO), come BSI, autorizzate dalle Autorità Regolatorie (RA .
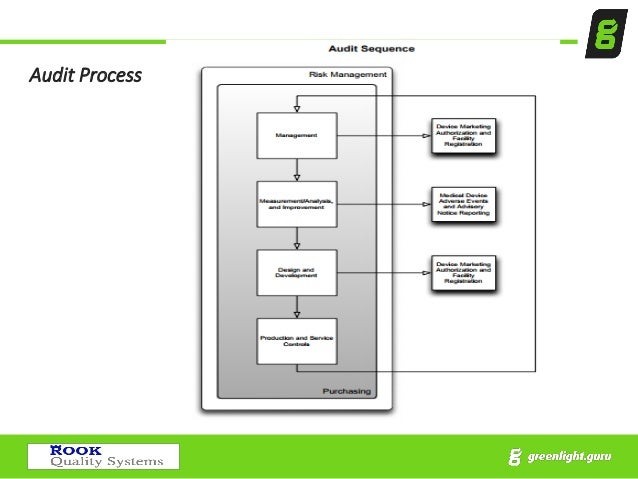
Technical Documentation Review Fees .MDSAP audit documentation includes update to MDSAP Companion Document.BSI is enthusiastic about the use of hybrid medical device audits.About BSI Group 13. Die in UK anerkannte Stelle - BSI UK (0086) führt Konformitätsbewertungen gemäß UKCA durch.In line with the changing world, BSI has reimagined the way we audit Medical Device Manufacturers.Medical device live and on demand webinars. Medical Device Coordination Group (MDCG) endorses guidance for .

Download the whitepaper >.