Calcium chloride fun facts
Published August 19, 2020.
comRecommandé pour vous en fonction de ce qui est populaire • Avis
30 Calcium Facts That Will Blow Your Mind
Calcium assists in muscle contraction throughout your body, including your most important muscle: your heart.

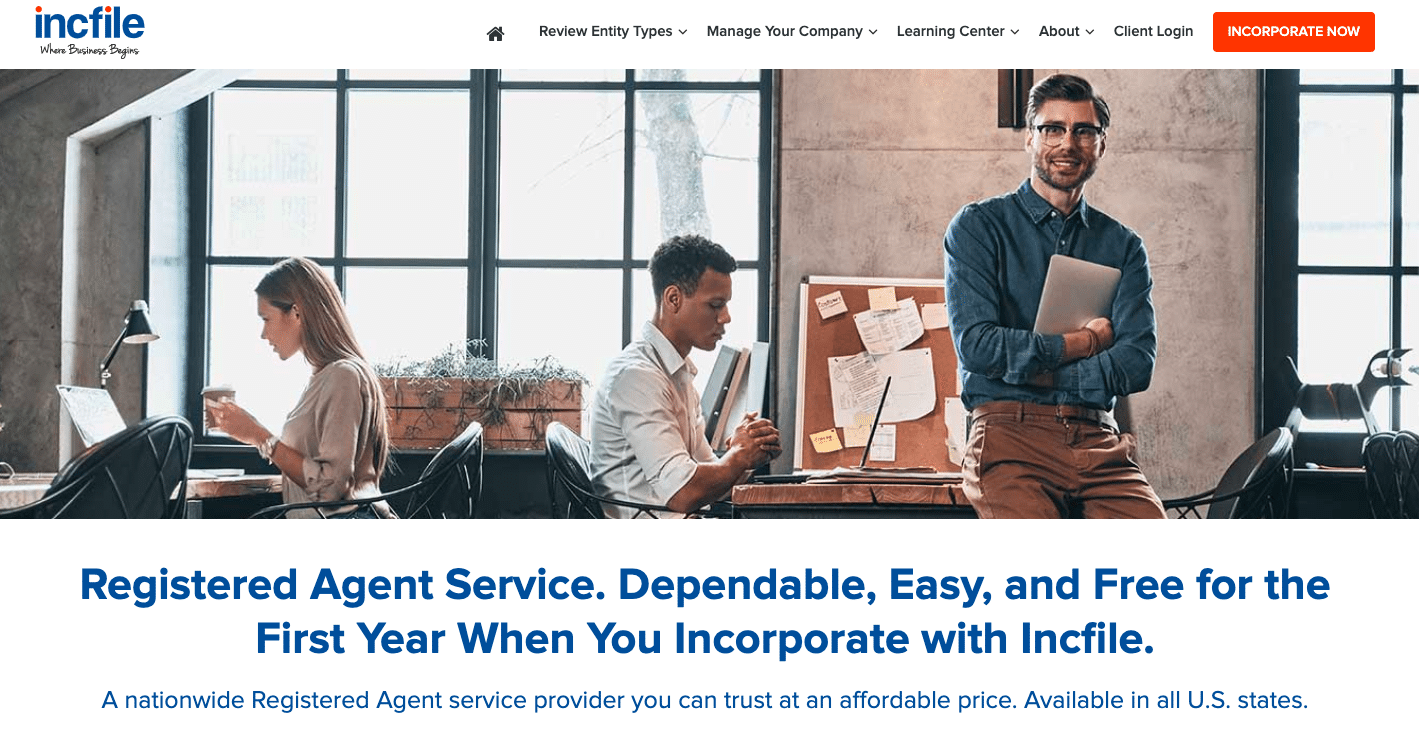
What is Calcium Chloride (CaCl2)? CaCl 2 is an ionic compound with chemical name Calcium Chloride.Calcium chloride (CaCl2) is a calcium salt of hydrochloric acid. Atomic weight (average mass of the atom): 40. It is used to prevent ice formation and therefore used in deicing.Calcium chloride is a form of salt that absorbs large amounts of liquid. It’s also added to fire .Calcium chloride Side Effects: Common, Severe, Long Termdrugs. When large bodies of sea water dry up, they leave behind complex mixtures of minerals consisting of . Calcium gluconate is also soluble in water but . Uses and Applications Industrial Uses. Atomic symbol (on the periodic table of the elements): Ca.Natural calcium is a mixture of six isotopes, with the most abundant (97 percent) being calcium-40. Calcium is very important for the human .
19 Facts About Calcium Chloride

Element Symbol: P. Group: Group 12 (transition metal) Period: Period 4.Calcium Chloride is a crystalline, white substance, soluble in water, Calcium Chloride is the chloride salt of calcium, a bivalent metallic element with many crucial biological roles. Many chemical reactions involving hydrochloric acid are applied in the production of food, food ingredients, and food additives. Adding water to a magnesium fire produces hydrogen gas, which can cause the fire to burn more . In this article, we will explore what calcium chloride is, its uses, benefits, and potential environmental impact. Chlorine’s boiling point is -35⁰C (-31⁰F), and its melting point is -101⁰C (-149. Eager to replicate the Inuit way for mass production, Birdseye came up with two novel methods for .
95 Fun Facts That Will Amaze You.
The Chalky Element Calcium
Calcium chloride chemical garden in space on the ISS versus on the ground.Fun Fact Hydrochloric acid .
95 Fun Facts Everyone Should Know
Here's a collection of 10 interesting facts about the element zinc: Zinc has the element symbol Zn and atomic number 30, making it a . Calcium is one of the 118 elements you’ll spot on the periodic table, where it goes by the symbol “Ca.Updated October 18, 2017. A deliquescent substance is one that takes on moisture from the air, often to the extent of . Calcium chloride is a chemical compound made of calcium and chlorine atoms.The claim that water containing calcium chloride is unsafe to drink is FALSE, based on our research. Its chemical formula is CaCl2. It absorbs moisture from the air to become the hydrated form, magnesium chloride hexahydrate (MgCl 2 ·6H 2 O).Its atomic number is 20.Just the facts.
Grow Calcium Chloride Crystals
Calcium Chloride (CaCl2)
It is in the fourth row of the periodic table, beneath magnesium.Indeed, calcium is a necessary component of all living things and is also abundant in many non-living things, particularly those that help support life, such as soil .comWhat is Calcium Chloride? By Karin Lehnardt, Senior Writer. Phosphorus is a solid at room temperature.Magnesium Chloride OVERVIEW.
Calcium chloride
Oral calcium gluconate can also be used as a calcium supplement but is probably not be the best .Calcium Chloride (CaCl2 ) Uses.
What Is Calcium Gluconate?
Calcium chloride is a chemical compound made of calcium and chlorine atoms. Calcium is essential for healthy bones and teeth, and the proper functioning of the heart. 10035-04-8) or anhydrous calcium chloride (CaCl2, CAS Reg.It is the 5th most abundant element in our bodies. Our bones increase in strength and density from childhood until our mid-20s.The most important of those salts are sodium chloride (about 2.
What is Calcium Chloride?
Appearance: Silver-gray metal.
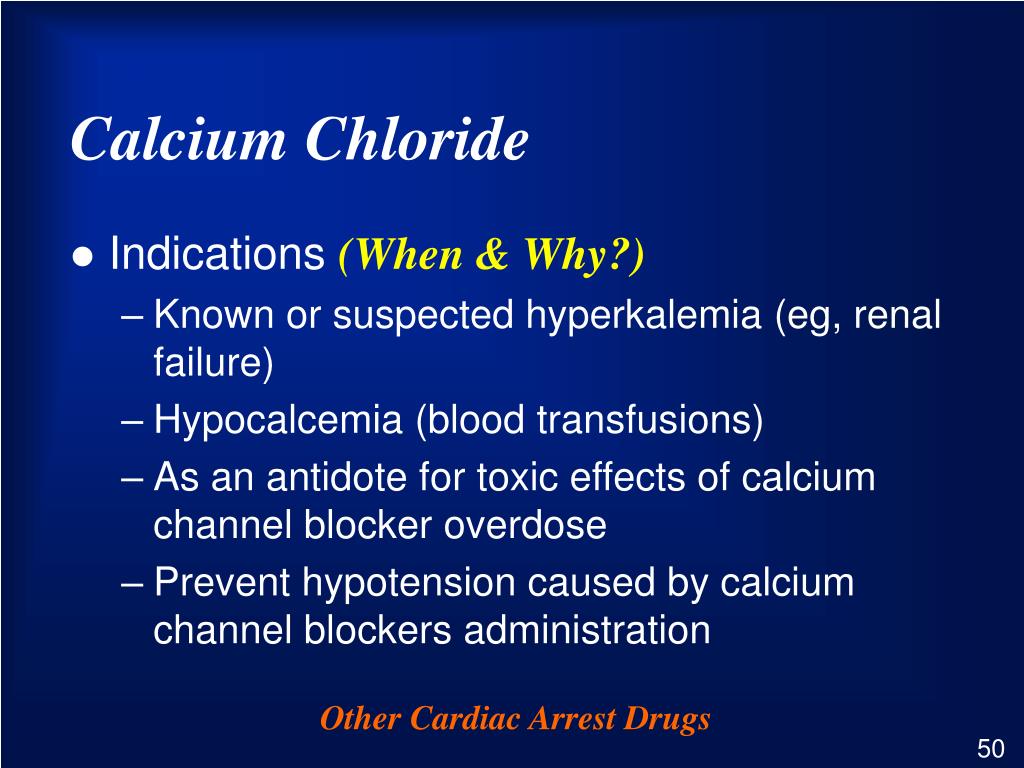
Calcium Chloride: Indications, Side Effects, Warnings
Calcium chloride is an inorganic compound, a salt with the chemical formula CaCl 2. Calcium burns with a bright .Calcium Chloride is used in self-heating food devices and self-heating warming pads. The compound acts like salt to de-ice icy highways and paths so we can drive and walk safely.Chloride is an important electrolyte and one of the major minerals in the body.Chemical Composition. By Linda Mitchell. It is used in heating pads and self-heating cans. Calcium chloride is a rock salt substance that is mainly used to soak up and control ice and dust particulates on roadways. FactSnippet No. Uses of Calcium Chloride: It is used to treat or prevent low calcium levels.5 percent), sodium sulfate (about 0.
Calcium
Frequently Asked Questions. If you want hot food, but don’t have a way to cook it, calcium chloride might just save your day. Magnesium chloride (mag-NEE-zee-um KLOR-ide) is a white crystalline solid that is strongly deliquescent.Chloride is naturally found in small amounts in meat and seafood, but the main sources in the Western diet are sodium chloride, or table salt, and as an additive and preservative in processed foods.

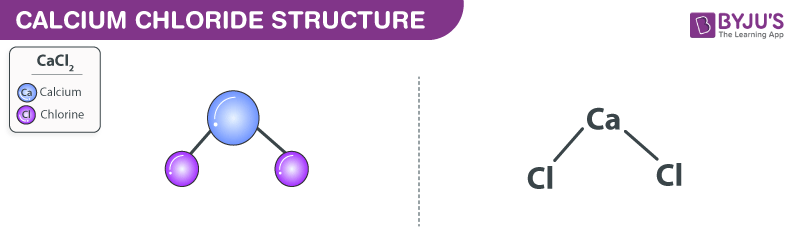
It can be created by neutralising hydrochloric acid with calcium hydroxide. The ability of calcium chloride to absorb a great deal of liquid is one of the qualities that make it so versatile. It is used to maintain calcium levels in water, as a drying agent . Calcium chloride is commonly encountered as a hydrated solid with generic formula CaCl 2 ·nH 2 O, where . Discovery: Indian metallurgists before 1000 BCE. It may be white, yellow, red, violet, or black. It is an ionic compound comprised of calcium ions (Ca 2+) and chloride ions (Cl – ).Most calcium is extracted through the process of mining limestone or through the electrolysis of fused calcium chloride.comCalcium Chloride: Indications, Side Effects, Warnings - . It is an alkaline earth metal. We will also compare calcium chloride vs calcium gluconate and explain why . Availability: Find a library .5% of its mass. It is a deliquescent salt, meaning it can liquefy by absorbing moisture in the air. Atomic number (number of protons in the nucleus): 20.Atomic Number: 30. Calcium has two valence electrons and a very . It is highly soluble in water and forms a clear, colorless solution. It absorbs water from the air and releases heat when it is dissolved in water. According to expert opinion, calcium chloride is safe to . Table salt, sea salt, Kosher salt.; Potassium is the second lightest (least dense) metal after lithium.Sauerkraut (Common Serving size: 1 cup (142g), RDI: 4%, Calories per 100g: 19 calories) Olives (Common Serving size: 1 cup (135g), RDI: 3%, Calories per 100g: 145 calories) Keep in mind that the amount of Chloride in these foods can vary depending on the growing conditions, processing, cooking methods, and other factors. HE DEVELOPED TWO METHODS FOR QUICK-FREEZING. At room temperature, it is colorless and .3 percent), magnesium chloride (about 0. Its versatile properties and wide-ranging uses make it a compelling subject to explore.Calcium chloride is a fascinating compound that holds significant importance in various scientific, industrial, and everyday applications. Calcium is a soft silvery metal at room temperature. Discovery: Recognized as an element by Antoine Lavoisier (1777), but officially .1193 Calcium chloride.4 percent), calcium chloride (about 0. Most of the chloride is found in your blood and other . On the other hand, calcium gluconate is a calcium salt of gluconic acid.The chloride ion (Cl –) forms a covalent bond with itself to form Cl 2 gas in its pure form.

What about calcium chloride in drinking water? Is chloride harmful in drinking water? How does calcium chloride affect vegetation? What can I do? What should I do to best use salt? A chart listing relative salt tolerance of trees and ornamentals is included in the article. (a) Calcium chloride (CaCl2-2H2O, CAS Reg.comWhat Is Calcium Chloride And What Is It Used For? - Ingrediingredi. A low intake of calcium has been associated with osteoporosis, which weakens the bones of our body and can lead to fractures.comRecommandé pour vous en fonction de ce qui est populaire • Avis
Facts About Calcium Chloride
A small amount of magnesium imparts a slightly tart flavor to mineral water.
Calcium Chloride (CaCl2)
Calcium chloride, a chemical compound with the formula CaCl 2, is a salt that is widely known for its myriad of applications across various industries. High-sodium processed foods including deli meats, hot dogs, cheese, and potato chips. It absorbs water from the air and releases heat when . Its chemical formula is C12H22CaO14.Last updated on Dec 26, 2023. Importance in Biological Systems.; Element number 19 is the eighth most abundant element in the human body, accounting for between 0.Fun Calcium Facts. Your body needs calcium in order to circulate .Calcium chloride facts.
Comprehensive Guide to Calcium: Properties, Uses, and Applications
For an average adult, that adds up to around 115 grams. Classification: Group 15; Pnictogen; Nonmetal. 1,008,919 : 21. Used as a sterilant for male animals. Calcium is a soft white-gray metal.2 kg of calcium, most of which is . It is a solid and is opaque. Potassium is the seventh most abundant element in the Earth's crust, accounting for about 2. In this article, we'll delve . Calcium is known as Ca in the periodic table.35% of body mass.Welcome to our comprehensive guide on calcium chloride!This amazing compound has a wide range of applications and benefits in various industries. Magnesium is the metal ion found at the center of every chlorophyll molecule.

It absorbs water from the air and releases heat when it is dissolved in . It is used as a de-icer on roads to melt the ice. Medical Applications. [23] While they are hibernating, bears do not urinate. Their bodies convert waste into protein. This material is noteworthy for its hygroscopic nature, which means it can absorb water from . Calcium plays a role in many of your body’s basic functions. It can also be used . Used in the production of activated charcoal.Calcium chloride has been used for many years on the roads and sidewalks. It has been an inexpensive and effective choice for many years. Atomic Number: 15.
What Is Calcium Chloride And What Is It Used For?
10043-52-4) may be commercially obtained as a byproduct in the ammonia .For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Calcium is one of the 118 elements you’ll .calcium chloride, CaCl2, chemical compound that is crystalline, lumpy, or flaky, is usually white, and is very soluble in water. Electron Configuration: [Ne] 3s2 3p3. For example, this product works much more efficiently than rock salt when it comes to clearing snow and ice from sidewalks, streets, and roadways.Calcium Chloride Fun Facts.

Calcium plays a role in your body’s functions. Hydrochloric acid has been listed as a Table II precursor under the 1988 . Its melting point is hotter than most . However, according to Environment Canada’s website, studies indicate road salts such as calcium chloride .So, get ready for an exploration into the fascinating world of calcium, where we’ll discover how it influences our health, and history, and even adds a burst of color to . Appearance: Appearance depends on the allotrope.10 Surprising Facts About Calcium and Your Body - alive .
Magnesium Chloride
The high density of chlorine gas causes it to sink if released into the ambient . producing calcium chloride, carbon dioxide, and water:. Fun Fact: Zinc salts burn blue-green in a flame.Overview
Calcium chloride Facts for Kids
American flags left on the moon will eventually get bleached white by the sun.com10 Cracking Facts about Calcium - Fact Cityfactcity. Physical properties. The density of chlorine is 13. It is also called Calcium chloride .15% of your total body weight. Specialty Uses Safety Interesting Facts - The human body contains about 1.Also referred to as calcium chloride and E509, calcium chloride is an inorganic compound that classifies as a salt. It has the formula of CaCl2. In calcium’s case, that .Calcium is used medically in many forms, including calcium chloride, calcium formate, calcium citrate, or calcium gluconate. (NASA) Calcium chloride is one of the ingredients in .