Calcium peroxide and water reaction

Calcium peroxide (CPO) (CaO 2) is one of the safest biological oxygen-generating materials [84], its high purity and slow-release characteristics are considered to be the most promising biological materials in the fields of tissue engineering [85].Auteur : Hefei Wang, Yongsheng Zhao, Tianyi Li, Zhen Chen, Yinan Wang, Chuanyu Qin
Calcium peroxide
Effect of calcium peroxide particles as oxygen-releasing
CaO2 has also been used in cancer therapies, such as photodynamic therapy.
The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide: H2 + O2 → H2O2.The results showed that CaO 2 could promote SMX elimination in O 3 system.78 times that in the control .
Manquant :
water reaction Magnesium peroxide is less soluble in water, so it reacts with water slowly, leading to a gradual release of oxygen.5H 2 O 2, SPC) and calcium peroxide (CaO 2, CP) (Sun et al., CuS/TAED/CP) process was tentatively employed for the degradation of sulfamethazine (SMT) in water. 331 - 337 , 10. Almost certainly.This study describes the enhanced photochemical degradation of emerging contaminants in water using a UV-activated calcium peroxide . Posted March 1, 2012.Magnesium, calcium, strontium and barium oxides react with water to form hydroxides: \[ MO(s) + H_2O(l) \rightarrow M(OH)_2(s) \label{14} \] All the oxides and hydroxides of the . The activation of hydrogen peroxide (H 2 O 2) by ultraviolet (UV) has garnered increasing attention. The product of this reaction is called a peroxide because oxygen is in the O2 − 2 form (hydrogen has a +1 oxidation state).
Manquant :
water reactionCytotoxicity of Bleaching Products: A Systematic Review
Hence, it is urgent to develop an effective technology for the remediation of 1,2-DCA-contaminated groundwater.
Polymers
Magnesium peroxide is less soluble in water, so it reacts .CPO contact with H 2 O and decomposes to produce H 2 O 2 ·H 2 O 2 is then decomposed into H 2 O and O .
It will certainly give off heat.They are considered as solid hydrogen peroxide (H 2 O 2) and slow oxygen-releasing compounds (ORCs), . Therefore, in this study, starch, a non-toxic, . In a 250 ml beaker, 5 g calcium sulfate was dispersed in 50 ml deionized (DI) water and 20 ml KOH (1 M).

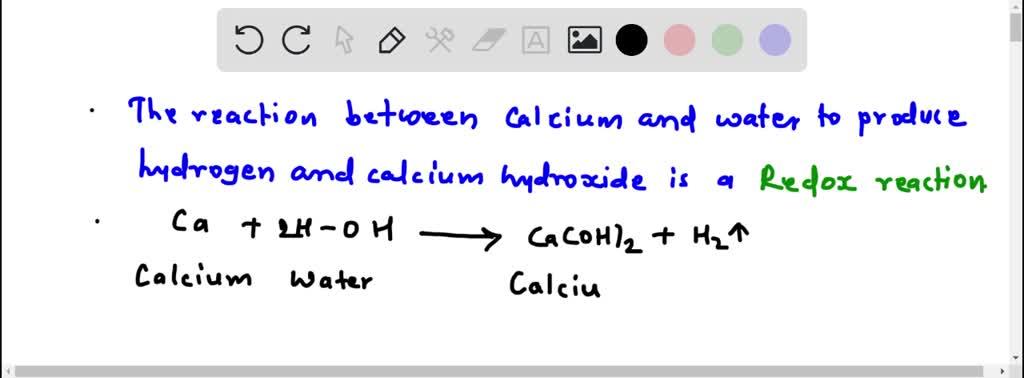
(i) Reaction between calcium oxide and water is a .
Manquant :
calcium peroxideEnhanced-oxidation of sulfanilamide in groundwater using
Furthermore, the nanoscale surface chemistry of . Calciphylaxis (kal-sih-fuh-LAK-sis) is a serious, uncommon disease in which calcium accumulates in small blood vessels of the fat and skin tissues. This Module addressed why it is difficult to observe a tidy pattern .Results for two months indicated that the dissolved oxygen (DO) concentration of the overlying water in the test zone was 3.Sulfamethoxazole (SMX) is a widely distributed emerging contaminant, which will bring serious harm to ecology and human health.

Additionally, Fe(II), an activator . However, the uncontrollability of oxygen release after immersion in water is a challenge.Activation of hydrogen peroxide (H2O2) to ·OH is considered to be one of the most potential methods for treating micropollutants in water.Calcium peroxide (CP)-based advanced oxidation processes for water decontamination have stimulated considerable attention due to their inherent merits. The products are initially going to be calcium peroxide and water, but I don't think the peroxide is very stable. Bubbles of hydrogen gas are given off, and a white precipitate (of calcium hydroxide) is formed, .Sodium percarbonate (SPO) and calcium peroxide (CPO) are two common oxygen-generating materials. Tests may include: Skin biopsy.The XRD results showed the production of two different polymorphs of calcium carbonate, CaCO 3 (calcite and vaterite), after exposure of calcium peroxide in water . Hydrogen peroxide was added to the reaction beaker in a molar ratio of 1:7 (CaSO 4: H 2 O 2) and the mixture . The oxygen is produced by the decomposition of hydrogen peroxide into oxygen and water. The balanced equation will appear above.A reaction scheme for the formation of the ROS on CaO 2 was also proposed and discussed. Herein, a novel tetraacetylethylenediamine (TAED) coupled CuS/CP (i. Similar questions.
does calcium oxide react with hydrogen peroxide?
The final products will be calcium hydroxide, oxygen and water. However, calcium peroxide (CPO) is purer and cheaper than magnesium peroxide. The method was tested in both laboratory and field conditions. Hydrogen peroxide was added to the reaction beaker in a molar ratio of 1:7 (CaSO
Application of calcium peroxide in water and soil treatment: A review
what kind of reaction is this. Of more interest was the finding that the CaO 2 which released the • OH and • O 2 − on the surface exhibited good transition properties compared with alkaline-earth metal peroxides of the same group (MgO 2 , BaO 2 ). Calciphylaxis . Hydrogen peroxide is also used .The CaO 2 powder quickly spreads in water and the CaO 2 particles produce calcium (Ca 2+) and peroxide (O 2 2−) ions on the particle sur-face, and the O 2 2− can combine .To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button., 2016; Zhou et al.Calcium peroxide (CaO 2), as one of the solid peroxides, has been increasingly used in contaminated site remediation due to its ability to release oxygen (O 2) and hydrogen peroxide (H 2 O 2). To determine if you have calciphylaxis, your doctor will review your medical history, assess your symptoms and do a physical exam. It is imperative to .
Fenton reaction using hydrogen peroxide (H 2 O 2) has been widely applied to achieve the in-situ chemical oxidation of contaminated soil and groundwater.Group 2 elements (beryllium, magnesium, calcium, strontium and barium) react oxygen.1 g/g TS (total solids), leading to 89 % and 54 % removal .
If it reacts with steam, the metal oxide is formed.Calcium peroxide (CaO2) has recently attracted much attention as an oxygen-releasing biomaterial for tissue engineering.environmental applications is calcium peroxide (CaO 2).
Calcium Peroxide
Calcification (Calcium Deposits)
Among them, CP can slowly release H 2 O 2 when .14,15 Previously, CaO 2 wasmostlyusedasanoxygenrelease compound (ORC), which could slowly decompose to release oxygen when in contact with water (reaction (10)).The chlorobenzene (CB) degradation performances by various oxidants, including hydrogen peroxide (H 2 O 2), nanoscale calcium peroxide (nCaO 2) and sodium percarbonate (SPC), activated with ferrous iron (Fe(II)) were investigated and thoroughly compared.In this study, the removal of sulfamethoxazole (SMX) and hydrocortisone (HC) from waste activated sludge (WAS) was explored using a dual oxidant strategy involving calcium peroxide (CaO 2) and peroxydisulfate (PDS).Degradation of 2, 4, 6-trinitrotoluene in water and soil slurry utilizing a calcium peroxide compound Chemosphere , 40 ( 2000 ) , pp.The hydrogen peroxide (H 2 O 2) produced in this reaction later breaks down into oxygen and water .Biodegradation can be enhanced by the existence of O 2. Use uppercase for the first . Calcification is a gradual accumulation of calcium in body tissue. SPO is an adduct of hydrogen peroxide and sodium carbonate.Calcium peroxide (CP) from the group of alkaline earth metal peroxides acts as an oxidizing agent for the degradation of organic dyes in water.Overview
Reactions of Main Group Elements with Water
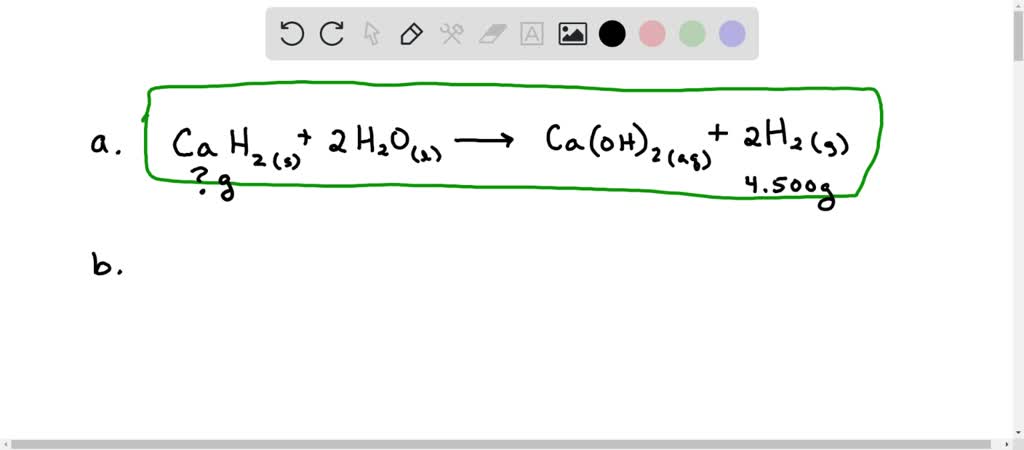
It is known that the commercially available CP has a relatively large particle size, which makes the reaction rate for pollution degradation relatively slow.Calcium peroxide nanoparticles were synthesized by the reaction of calcium sulfate and potassium hydroxide.The Fenton reaction generates hydroxyl radical (HO), which possesses a high oxidation potential .The general reaction of calcium, strontium, and barium with water is represented below, where M represents calcium, strontium, or barium: M(s) + 2H2O(l) M(OH)2(aq) +H2(g) .Calcium superoxide (Ca(O 2) 2) was synthesized by disproportionating calcium peroxide diperoxyhydrate. The reactants are calcium hydroxide and hydrogen peroxide.
Calcium peroxide (CaO 2) is one of the most common oxygen sources for these types of materials because it generates oxygen, hydrogen peroxide, and calcium .Itisknown [5] that nexchange reaction between Ca(OH)2 andII202 takes place in hydrogen peroxide 301utions; this leads toformation of hydrates orp rhydrates, depending on the .
The reaction of calcium hydroxide with hydrogen peroxide vapor
calcium oxide reacts with water to form calcium hydroxide.