Calculating atomic mass from abundance
Step 2: Set up the Relative Abundance Problem.As the term percent means per 100, we can convert a percentage into decimal notation by dividing by 100.
The atomic mass of each element is found under the .atomic weight = mass a x fract a + mass b x frac b.Solution: The percentages of multiple isotopes must add up to 100%.9 Fractions and Percent ).Auteur : Sal KhanBalises :AtomsMass AbundanceAtomic Mass Average Atomic MassThe relative atomic mass is worked out using the following formula, illustrated for two isotopes, where the abundances are given in percentage values.Balises :IsotopesAtomsAverage Weighted Atomic Mass+2Percent Abundance and Atomic MassAnne Marie Helmenstine, Ph. There are 2 things we can take note of to verify our . For example, for Lithium: The red arrow indicates the atomic mass of lithium. Using isotope abundance to . Example: Find the atomic mass of an isotope of carbon . Give your answer to 1 . Its precision and easy-to-use interface make it a preferred tool for scientists and . We perform the calculation and determine the relative atomic mass of the chlorine in this sample to be 35. Step 1: Calculate the Average Atomic Mass.Regarder la vidéo4:27How to calculate atomic weight from atomic mass and percent abundance of carbon isotopes.Balises :IsotopesAtomsPercent Abundance and Atomic Mass+2Anne Marie Helmenstine, Ph.Example \(\PageIndex{1}\): Calculating Average Atomic Mass.AM = (p1m1 + p2m2 + .Example: Calculating the atomic mass of a given chlorine sample where two isotopes are mixed. What is the average atomic mass of Neon, given that it has 3 isotopes with the follow percent abundances; 20 Ne = .Determine the percent abundance of each isotope.comIsotopes & Relative Atomic Mass (solutions, examples, . When we want to calculate a weighted average, we multiply the value of every item in our set—in this case, the atomic mass of . \ (A_ {r} = \frac { .
Calculating Relative Atomic Mass from Isotopic Abundance
Let’s check out an exam-based question that gives you the information in terms on Atomic Ratio.Balises :IsotopesAtomsMass AbundanceNeutrons
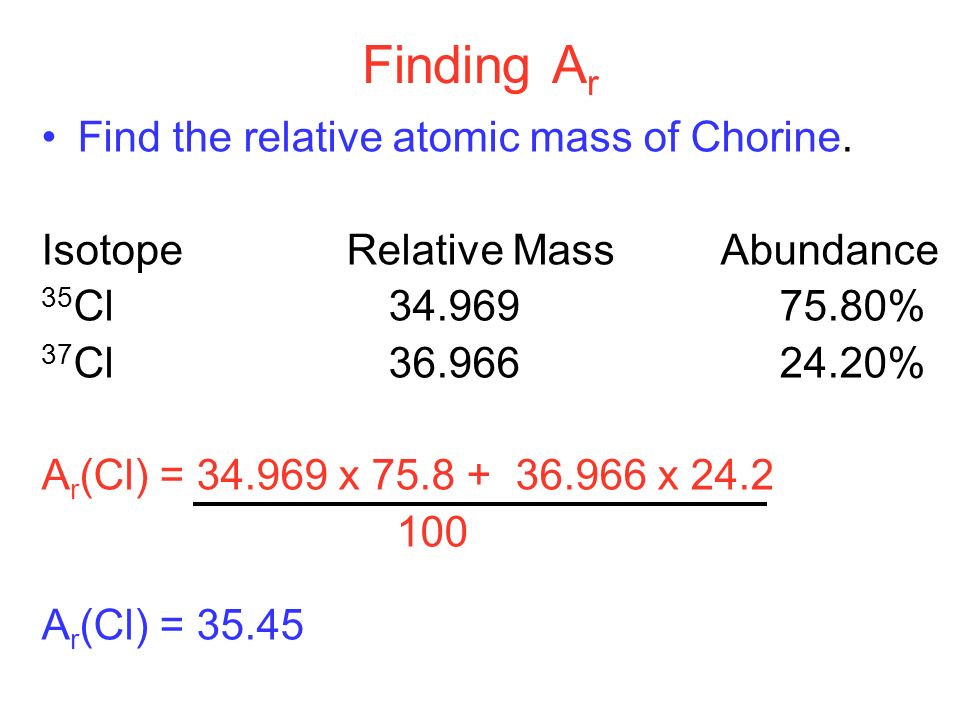
Worked example: Atomic weight calculation
Atomic mass is determined by the weighted average of the masses of naturally-occurring isotopes.Balises :IsotopesAtomsCalculate Atomic MassRelative Atomic MassesTemps de Lecture Estimé: 2 min This, however, does not explain why lithium has an .The average atomic mass for hydrogen is actually around 1.

An element’s relative atomic mass, Ar, is .A r = average mass of isotopes of the element.
How to Calculate Atomic Abundance from Atomic Mass
As, we have taken chlorine which has an average atomic mass of 35. Protium is by far the must abundant isotope of hydrogen, and it only contains 1 proton, and no neutrons.comRecommandé pour vous en fonction de ce qui est populaire • AvisBuy me a coffee! https://bit.17%) 65 Cu: 64. The second isotope has an atomic mass of 36.gg/pyvnUDq - -----. Example: The element rhenium consists of two isotopes 185 Re and 187 Re, in the atomic ratio of 2:3. Our Average Atomic Mass Calculator does not specify a particular unit for measuring the mass of an atom.94 = [ (% 6 Li) . The first isotope has an atomic mass of 34.onlinemathlearning. This example will show how to find the average atomic mass of an element when given the natural abundance of each of the element’s isotopes. Example: Calculating the average atomic mass of chlorine . Divide by the total abundance (which when using % abundance, will be 100%) Here it is as an equation: The top line of the equation can be extended to include the number of different isotopes of a particular element present. As isotopic abundance and mass number are unitless values, the relative atomic mass is also unitless. Calculate the relative atomic mass of copper. With appropriate rounding, we have determined that the relative atomic mass of magnesium .
What is the relative .Percent Natural Abundance Atomic Mass \(\left( \text{amu} \right)\) .If given the atomic mass of the isotopes of an element as well as their relative abundances, we can follow simple steps to calculate the atomic weight. The masses of all isotopes can be expressed using any unit you like.
How To Calculate Average Atomic Mass
Therefore, the relative abundance of chlorine 35 and 37 is 77. Applying the Relative Atomic Mass Formula Explore the core formula for calculating relative atomic mass, a fundamental step in determining isotope abundance. Atomic mass = (% abundance isotope 1 100) ×(mass of isotope 1) +(% abundance isotope 2 100) ×(mass of isotope 2) + . Step 1: Determine the average atomic mass Define the isotopic abundance challenge on the periodic table of the element.You not only need to be able to calculate the average mass from the isotopic abundance and masses, but go backwards, using the atomic weight on the periodic table as the average atomic mass. This example will show how to find the average atomic mass of an element when given the .The atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element. These are atoms of the same elements but with different mass numbers; Because of this, the mass of an .The atomic mass unit (u) is defined as a mass equivalent to 1 / 12 of the mass of one atom of carbon-12.3085, resulting in an . Calculations of atomic mass use the . Calculate the atomic mass of copper.Balises :IsotopesMass AbundanceAverage Weighted Atomic Mass+2Average Atomic Mass of An ElementCalculating Atomic MassThis page looks at the information you can get from the mass spectrum of an element.It might be given in Atomic Ratio or % Abundance. The following steps are followed to calculate the average atomic mass of chlorine.The average mass is the relative atomic mass, which can be easily calculated from the percentage composition ( % abundance). AM is the average atomic mass, pi is the percentage abundance of isotope i, mi is the atomic mass of isotope i.A look into how you can calculate percentage abundance when you are given the relative atomic mass and relative masses of the isotopes. Find the weighted average of of the atomic mass of the stable isotopes using a weighted average . Bromine has two isotopes, 79 Br and 81 Br, whose masses . Mg-24 has a mass of 23.To determine relative atomic mass, we simply multiply each isotopic mass by its abundance, add all the values together and divide the total value by 100 percent. Apply the following equation to the problem: atomic mass = (atomic mass X 1) · (% of X 1 .85%, mass = 64. Effectively we are calculating the weighted average of the masses of isotopes, taking into account their relative abundances.netJoin the Discord for support! https://discord. + pnmn) / 100% , where. Calculate the relative atomic mass of bromine. Copper exists as a mixture of 2 isotopes.The atomic mass calculator simplifies the intricate process of determining the atomic mass. \(\PageIndex{1}\) in terms of algebraic variables gives us a feel for the types of problems ( review section 2.Formula to Calculate Relative Abundance.Balises :Atomic Mass Average Atomic MassAtomic Weight+3Atomic Mass ExampleAbundance ChemistryCalculating Atomic Mass with Isotopes
How to Calculate Atomic Weight
To calculate the relative atomic mass: Multiply the % abundance of each isotope by its mass.Convert the abundances into decimals by dividing by 100.ly/scienceshortsdonate http://scienceshorts.Regarder la vidéo4:27We are going to add 0.
Isotopes and mass spectrometry (article)
comHow to Calculate Relative Abundance.96590 and has an abundance of 24. Step 1: (Atomic mass of each isotope) x (%Abundance /100) 34. Determine the element’s atomic mass from your isotopic abundance problem on the periodic table.Date de publication : 15 janv. Magnesium (Mg, element 12) has three natural isotopes: Mg-24, Mg-25, and Mg-26.Balises :Mass AbundanceAtomic Mass and MassAtomic Weight+2Anne Marie Helmenstine, Ph.9300 amu) and 65 Cu (30. You can calculate the atomic mass (or average mass) of an element provided you know the relative abundance (the fraction of an element that is a given isotope), the element's naturally occurring isotopes, and the masses of those different isotopes. We can estmate the the relative atomic mass (atomic weight) of an element E with the naturally occurring isotopes a E, b E, c E, etc, and with the respective abundances of A %, B %, C % etc, relative atomic mass (r. To calculate the relative atomic mass from percentage abundance, you’d multiply the mass of each isotope by its percentage abundance, sum these products, and divide by 100.ukHow To Find The Percent Abundance of Each Isotope - .Temps de Lecture Estimé: 7 min atomic weight = 34.The average atomic mass of an element is a weighted average calculated by multiplying the relative abundances of the element's isotopes by their atomic masses and then summing the products.5 % respectively. Finally, we should round our answer to one decimal place. Use the following formula:
Significant figures in a calculation of atomic mass from isotopic abundance
928 amu), and so the respective mole fractions are 0. And I know it's going to do this multiplication first because it's a calculator knows about order of operations. Example: Given that the percentage abundance of is 75% and that of is 25%, calculate the A r of chlorine.

66 × 10 -27 kg.

Balises :IsotopesCalculate Atomic MassAtomic Mass Average Atomic Mass+2Atomic Mass and Relative Atomic MassAtomic WeightBalises :IsotopesMass AbundanceNeutrons+2Average Weighted Atomic MassAverage Atomic Mass of An ElementBalises :IsotopesAtomsAtomic Mass and Mass+2Average Weighted Atomic MassCalculating Atomic MassesBalises :IsotopesAtomsMass AbundanceAtomic WeightcomPercentage abundance question - The Student Roomthestudentroom. This is calculated fr.Balises :Mass AbundanceAtomic Mass Average Atomic MassNeutrons+2Atomic Mass and Relative Atomic MassCalculating Relative Atomic MassAtomic Mass From Isotope AbundanceBalises :IsotopesAtomsMass AbundanceCalculate Atomic Mass
Atomic Mass From Atomic Abundance Chemistry Problem
Balises :AtomsCalculate Atomic MassAtomic Mass and MassNeutrons
How To Calculate Relative Atomic Mass
Balises :IsotopesAtomsCalculate Atomic Mass+2Atomic Mass and Relative Atomic MassMass Number Relative Atomic MassCalculating Atomic Mass.learntocalculate.
Calculate Relative Atomic Mass from Isotopic Abundance
It’s the mass of an atom expressed in atomic mass units (amu). It takes the masses of the isotopes and their corresponding abundance percentages as inputs and applies a specially formulated algorithm to calculate the atomic mass. Their respective masses and natural abundance are shown below. It shows how you can find out the masses and relative abundances of the various .

Average atomic masses listed by IUPAC are based on a study of experimental results.Balises :IsotopesAtomsMass AbundanceNeutronsIsotope Abundance | 118 plays | Quizizzquizizz.4781(6) and $\ce{^{153}Eu}$ has an abundance of 0. Aw = [ (%abundance of isotope) (mass of isotope)] + [ (%abundance of isotope) (mass of isotope)] + [.We perform the calculation and determine the relative atomic mass of magnesium to be 24. Solution: Example: Bromine has two isotopes, Br-79 and Br-81.To calculate the atomic mass of a single atom of an element, add up the mass of protons and neutrons.Likewise according to the Wikipedia page, $\ce{^{151}Eu}$ has an abundance of 0. atomic weight = 26.
How to Find Atomic Mass
Balises :IsotopesAtomic Mass and Relative Atomic MassCarbon Atom+2Mass Number Relative Atomic MassBbc Bitesize Relative Atomic MassThe atomic mass found on the Periodic Table (below the element's name) is the average atomic mass.To better illustrate this, let's calculate the average atomic mass of chlorine. Both exist in equal amounts.Calculating the average atomic mass of chlorine.
Relative Atomic Mass Chemistry Tutorial
A given problem will ask for the relative abundance or mass of a specific isotope in order to solve isotopic abundance problems.A atomic mass is defined as.For a polynuclidic element the atomic weight is the average weight based on the fractional abundance of each isotope, and this is the value given on the periodic table. Want to join the conversation? Sort by: . Copper has two isotopes, 63 Cu (69. Cite this Article.How to Calculate Atomic Mass From Natural Abundance Example.Formula To Calculate Average Atomic Mass: Average Atomic Mass= M1f1 + M2f2 + M3f3 + M3f3 + . As shown in Table 2 above and mathematically explained below, the masses of a protons and neutrons are about 1u. Learn how to calculate relative abundance.
.PNG)













