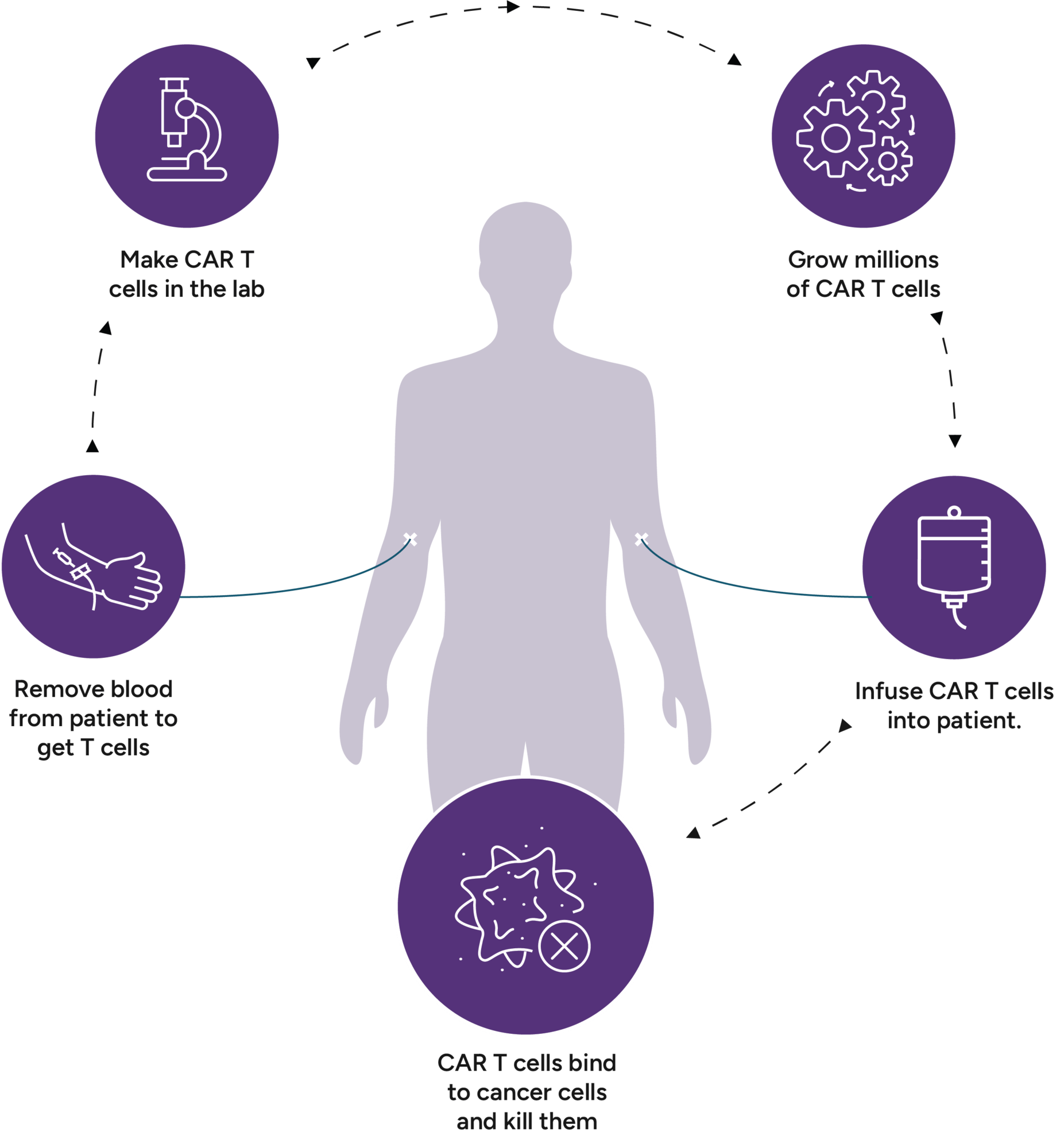
Car t cell therapy rrmm
2021 Dec; 13(23): 5886.CRB-402 (NCT03274219) is a phase 1 clinical trial evaluating bb21217 in patients with relapsed or refractory multiple myeloma (RRMM) [47, 48].LCAR-B38M is a structurally differentiated CAR-T cell therapy containing a 4-1BB co-stimulatory domain and 2 BCMA-targeting single-domain antibodies designed to confer avidity.
Manquant :
rrmm January 25, 2021.Long-term analysis of cellular immunity in patients with RRMM
Data 2-years post LPI (~30-month median follow-up . Ide-cel, a BCMA-directed CAR T cell therapy, showed frequent, deep, and durable responses in heavily pretreated pts with RRMM in .Chimeric antigen receptors (CAR) are engineered fusion proteins designed to target T cells to antigens expressed on cancer cells. 2020;38[suppl] [abstract 8503]).
Although an activated immune response . Here we report updated results from a longer median duration of follow-up of 18 months (mos). We evaluated the real-world outcomes of patients treated with standard of care ide-cel under the commercial FDA label.FCARH143 is a fully human BCMA-targeting CAR T-cell therapy that is formulated in a 1:1 ratio of CD4 + to CD8 + CAR T cells for infusion and expresses a truncated non-functional human epidermal .

comThe next-generation CAR-T therapy landscapenature. February 1, 2021. These CAR T cells encode humanized or murine scFvs, or camelid heavy chain .Treatment options for patients with RRMM are limited, and we are pleased that Abecma is the first CAR T cell therapy to be approved for earlier use as a treatment option to address the unmet needs of these patients. Safety and efficacy results of the CARTBCMA-HCB-01 multicenter clinical trial for .
Efficacy and Safety of CAR-T Therapy for Relapse or Refractory
8016 Background: Patients (pts) with RRMM previously exposed to immunomodulatory agents, proteasome inhibitors (PIs), and CD38 antibodies (mAbs) have poor outcomes with subsequent treatments.Balises :T CellsCAR TRelapsed Multiple MyelomaPeter Sidaway More clinical trials are needed in order to come up with more effective treatments of high-risk RRMM.Here, we aimed to explore potential biomarkers that might correlate with cytopenia following CAR T-cell therapy in RRMM.uchicagomedicine. Adam Samuel Sperling, Benjamin Avi Derman, Sarah Nikiforow, Soo-Yeon Im, Shuntaro Ikegawa, Rao H. T-Charge, an innovative platform that reduces manufacturing time to <2 days and preserves T cell stemness, results in robust expansion and prolonged CAR T cell persistence.govLong-term outcomes following CAR T cell therapy: what we . Herein, we conducted this .Background: Ide-cel, a BCMA directed CAR T-cell therapy, was FDA approved 3/26/2021 for the treat-ment of RRMM after 4 prior lines of therapy.In RRMM, the median overall survival (OS) of pts with RRMM who progressed after exposure to ≥3 prior therapies is ~13 mo, indicating a high unmet need.Investigations on the therapeutic use of CAR-T cells remains the “hot topic” in RRMM also in 2020/2021; several excellent and comprehensive reviews have been .comCAR T-cell therapy for multiple myeloma: state of the art and .orgPredictive factors of early progression after CAR T-cell .
RRMM: Considerations for Using CAR-T in Clinical Practice
European Hematology Association 2022 Congress; Jun 11, 2022; Vienna, AT.
CAR T therapies in multiple myeloma: unleashing the future
T cells engineered to express chimeric antigen receptors (CARs) with tumor specificity have shown remark-able success in treating patients with hematologic . Clinical evidence for immune-based strategies in early-line multiple myeloma: current challenges in decision-making for subsequent .CAR-T cell therapy in multiple myeloma: Current limitations .Ide-cel, a BCMA targeted CAR T cell therapy, showed promising tolerability and efficacy in RRMM pts in the phase I CRB-401 study ( NEJM 2019;380:1726).ashpublications.
Manquant :
rrmmCAR T-Cell Therapy, a Breakthrough Treatment for Cancer Patients
CELLULAR IMMUNOTHERAPIES: EARLY PHASE AND INVESTIGATIONAL THERAPIES BMS-986393 (CC-95266), a G Protein-Coupled Receptor Class C Group 5 Member D (GPRC5D)-Targeted Chimeric Antigen Receptor (CAR) T-Cell Therapy for .
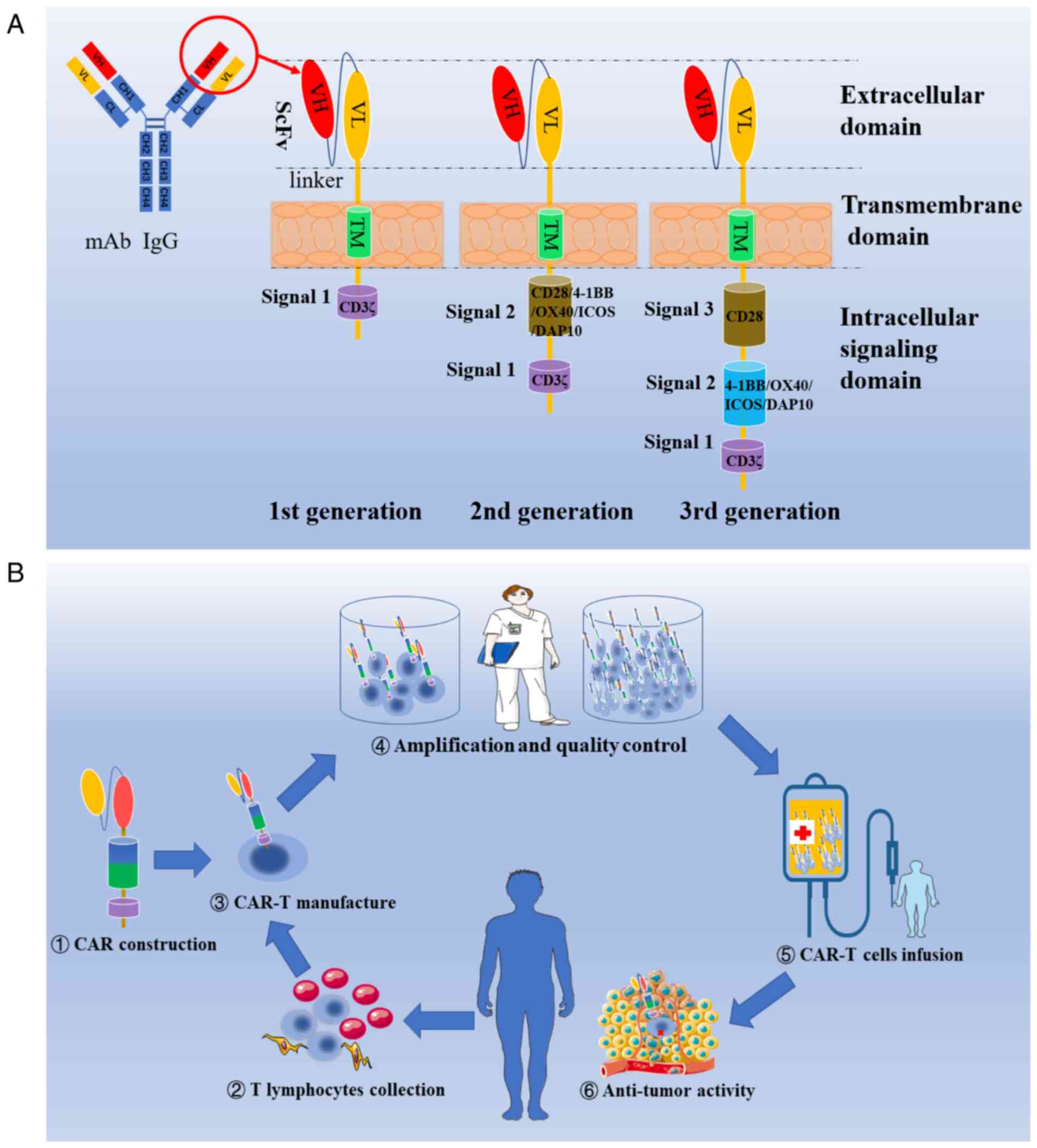
With CAR T-cell therapy a patient’s T cells are implanted with a new receptor that makes the T cell super potent and able to recognize tumor cells.Blood 142 (2023) 219–221 The 65th ASH Annual Meeting Abstracts ORAL ABSTRACTS 704. Published: 22 March 2023.1 million grant to enable University of California San Diego School . Considerations for where CAR T-cell therapy may fit into the standard of care for relapsed/refractory multiple .
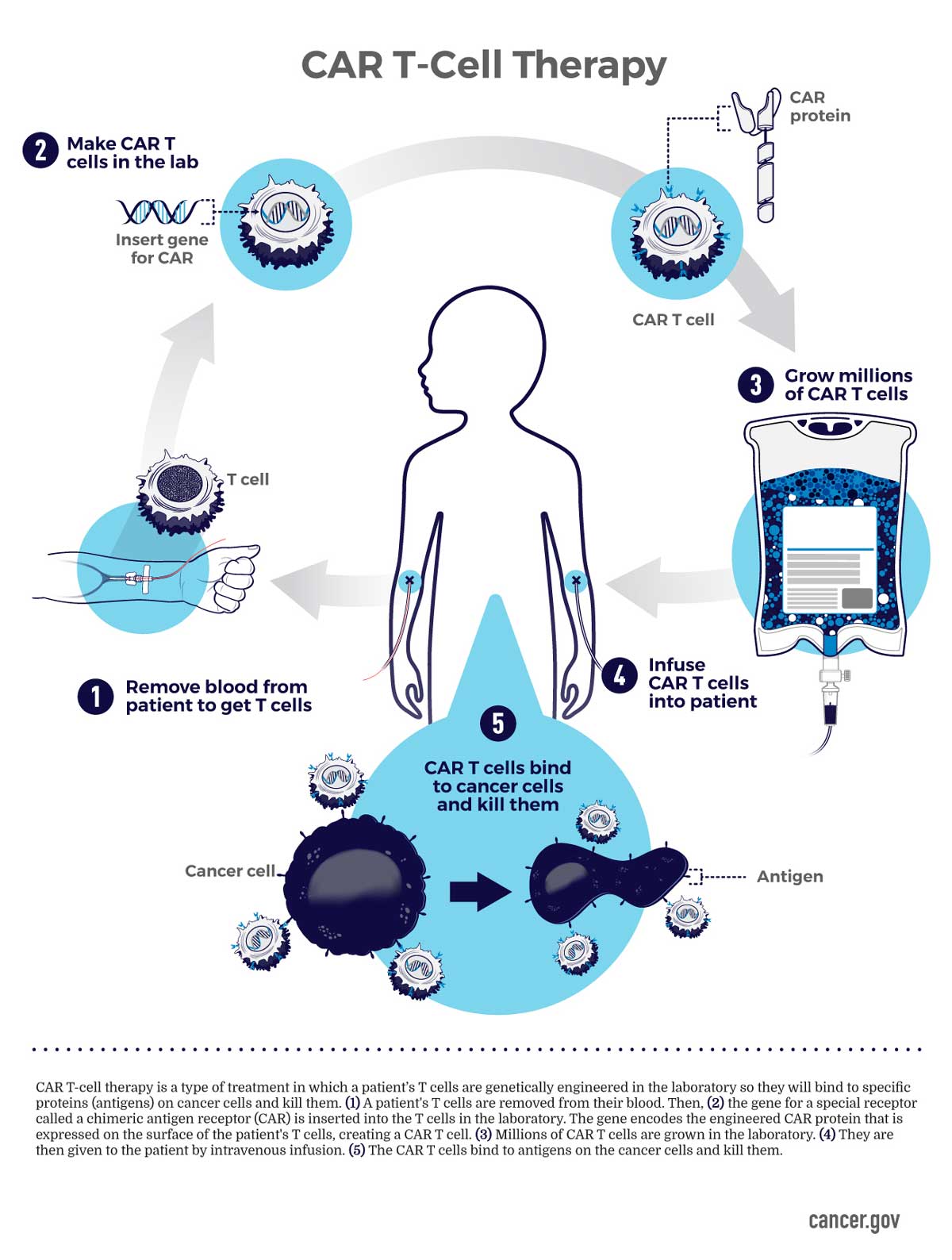
Idecabtagene vicleucel (ide-cel, also called bb2121), a B-cell maturation antigen–directed chimeric antigen receptor (CAR) T-cell therapy, has shown clinical activity with expected CAR T-cell .

Perez-Amill L, Suñe . 21 The median lines of prior therapies was 6 (range, 4-17), and 3 patients (14%) underwent autologous stem cell transplantation before CAR T-cell therapy. Outcomes are currently available from a total of six studies with a .Here we report the rational design and optimization of bispecific CAR-T cells with robust activity against heterogeneous multiple myeloma (MM) that is resistant to .The most promising developments include novel immunotherapeutic approaches such as chimeric antigen receptor (CAR) T-cell therapy and bispecific .On November 23, 2021, the California Institute for Regenerative Medicine (CIRM) governing board approved a $4.CAR T-Cell Therapy for RRMM: Measuring Outcomes.BCMA-targeted CAR T cell therapy for RRMM.8042 Background: Ide-cel, a BCMA directed CAR T-cell therapy, was FDA approved 3/26/2021 for the treatment of RRMM after 4 prior lines of therapy. Idecabtagene vicleucel (ide-cell, bb2121) was the first CAR-T cell product for RRMM .
CAR T-cell therapy in multiple myeloma: more room for improvement
Methods: Ten US academic centers con-tributed data to this effort independent of the .Most patients with relapsed and/or refractory multiple myeloma (RRMM) will have a response to anti-B cell maturation antigen (BCMA) chimeric antigen receptor .Newer CAR T-cell therapies, bispecific antibodies, and novel small molecules look promising.
Ciltacabtagene autoleucel (cilta-cel) is a CAR-T cell therapy with two B-cell maturation antigen–targeting single-domain antibodies.Disease characteristics were notable for 36% with high-risk FISH findings as defined by del(17p), t(4;14), and t(14;16), 50% extramedullary disease, 27% R-ISS stage III disease, and 30% with high bone marrow plasma cell burden (≥50%) prior to ide-cel infusion. Efficacy and safety of ARI0002h, an academic BCMA-directed CAR-T cell therapy with fractionated initial therapy and booster dose in patients with relapsed/refractory multiple myeloma.Chimeric antigen receptor T (CAR-T) cell therapy exhibits remarkable efficacy against refractory or relapsed multiple myeloma (RRMM); however, the immune deficiency .Balises :CAR TPublish Year:2020Understanding CAR T-Cell Therapy.Context: Cilta-cel, a CAR-T cell therapy with 2 BCMA–targeting single-domain antibodies, led to early, deep, and durable responses in heavily pretreated patients with RRMM in the phase 1b/2 CARTITUDE-1 study (NCT03548207).B-cell maturation antigen (BCMA)-targeted chimeric antigen receptor (CAR)-T cell therapy revolutionized treatment of relapsed and/or refractory multiple myeloma (RRMM) [1,2,3,4].The deep and durable responses with manageable safety profiles led to US Food and Drug Administration regulatory approval of idecabtagene vicleucel (ide-cel) .Introduction: Chimeric Antigen Receptor (CAR) T cell therapies directed against B-cell maturation antigen (BCMA) have demonstrated compelling clinical activity and manageable safety in subjects with relapsed and refractory Multiple Myeloma (RRMM). On-target off-tumor TRAEs, all G1/2, occurred in a minority of pts.Yan et al reported a phase 2 study of combined treatment with anti-CD19 and anti-BCMA CAR T cells in 21 RRMM patients.Balises :T CellsCAR TRelapsed Multiple MyelomaPublish Year:2021Anti-B cell maturation antigen (BCMA) chimeric antigen receptor (CAR) T-cell therapy seems to be a promising approach to treat RRMM patients. T cells engineered to express chimeric antigen receptors (CARs) with tumor specificity have shown remarkable success in treating patients with . CAR T cells are now an . Quinn, David Pearson, Dexiu Bu, Methods We prospectively collected . The approval was based on the phase II KarMMa trial, which included 128 subjects with a median of 6 prior lines of therapy. Long manufacturing time and high clinical demand limit access. For relapsed/refractory multiple myeloma (RRMM), preclinical evaluations of CAR-T therapy have shown promising efficacy, thus various active clinical trials are under way.
Manquant :
We remain committed to researching and developing innovative therapies to transform patient lives with serious diseases .comRole of CAR T Therapies in Newly Diagnosed Multiple . Oral abstract #S103.In this review, we summarize the current overview of CAR-T cell therapies in RRMM in 2021 with various targets for CAR-T cells and their efficacy, safety, and .Ide-cel, a BCMA-directed CAR T cell therapy, showed a favorable safety profile and frequent, deep, durable responses in heavily pretreated pts with RRMM (Raje et al.Balises :T CellsCAR TRelapsed/Refractory Multiple Myeloma in 2020/2021 and Beyond
Balises :Publish Year:2020Bcma Therapy MyelomaBcma Car-T Multiple MyelomaorgRecommandé pour vous en fonction de ce qui est populaire • Avis
CAR T cells show superiority over standard therapies for RRMM
Balises :Publish Year:2021 In pre-clinical studies, this academic CAR-T has demonstrated potent in vitro and in vivo activity. Earlier results from LEGEND-2 (NCT03090659), a first-in-human phase 1 study using LCAR-B38M CAR-T cells in 74 pts with RRMM conducted in 4 hospitals in . Prabhala, Diego Hernandez Rodriguez, Yifang Li, David S.Balises :T CellsBcma Therapy MyelomaBCMA-directed
CAR-T cells for the treatment of relapsed/refractory multiple

Eighty-four percent of the cohort . Methods: Ten US academic centers contributed data to this effort . It’s a one-time treatment and, if .
Combining CAR T cells effective in RRMM
BCMA-targeting CAR T-cells are currently being evaluated in several trials.Two BCMA-directed chimeric antigen receptor (CAR) T cell products have been approved for the fifth-line treatment of relapsed and/or refractory multiple .
Relapsed/Refractory Multiple Myeloma: How Treatment Changes
Updated phase I study results of PHE885, a T-Charge manufactured BCMA-directed CAR-T cell therapy, for patients (pts) with r/r multiple myeloma (RRMM).The emergence of B-cell maturation antigen (BCMA)–directed chimeric antigen receptor T-cell (CAR T) therapy is one of the most significant advancements in .CRS and ICANS-type neurotoxicity were mostly low-grade, with increased G ≥ 3 events at the 300 and 450 × 10 6 CAR T-cell doses.