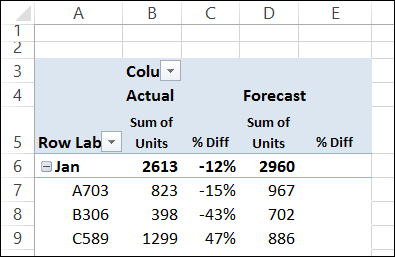
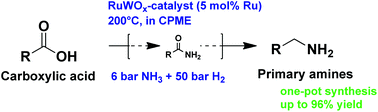
Carboxylic acid plus amine

The structure is the product of a carboxylic acid (the R R -portion) and an alcohol (the R′ R ′ -portion).Balises :Detailed AnalysisCarboxylic Acids ReactionSimple Carboxylic AcidsJust as the acid strength of a carboxylic acid can be measured by defining an acidity constant K a (Section 2-8), the base strength of an amine can be measured by defining an analogous basicity constant K b.
acid anhydrides with ammonia or primary amines
So in the first instance you get ethanoic acid and an organic compound called an amide.Balises :Carboxylic Acids ReactionCarboxylic Acid Mechanism So ethanamide tells you that you have a two-carbon chain that includes a C=O attached to an N atom, i.
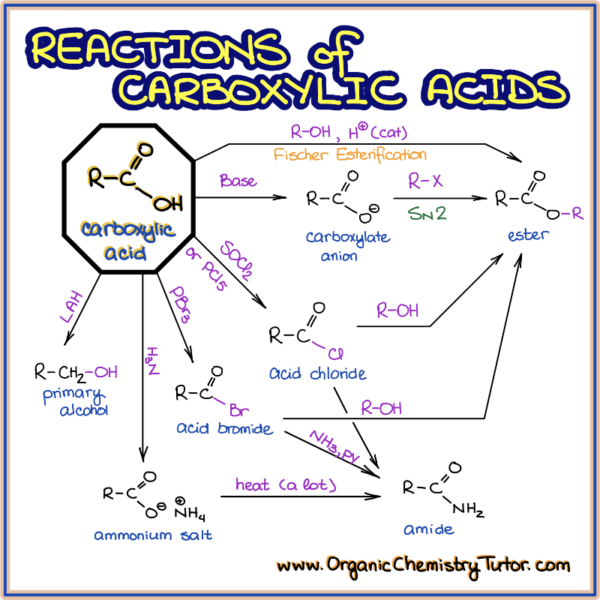
Balises :Carboxylic Acid MechanismPreparation of AmideAcid in Fischer Esterification Esters can be cleaved back into a carboxylic acid and an alcohol through reaction with water and a catalytic amount of strong acid. The conditions required for amide hydrolysis are more extreme than those required for the hydrolysis of acid chlorides or esters, but the mechanisms are similar .The direct conversion of a carboxylic acid to an amide is difficult because amines are basic and tend to convert carboxylic acids to their highly unreactive carboxylates. So, DCC has two main functions; 1) getting rid of the acidic proton, 2) converting the oxygen into a good leaving group. The amide coupling is a robust and popular reaction used frequently in chemical synthesis. Acide carboxylique. In addition, a suitable mechanism for the reaction pathway including possible side reactions was presented. Decarboxylation reaction. The amide functional group is to amines as esters are to alcohols. The amine is removed and replaced .Amides can be hydrolzyed to a carboxylic acid and ammonia or amine by heating the reaction under acidic or basic aqueous solutions. identify the amide linkage . The general rule for naming amides is that you replace the ending oic acid of the acid name with the ending amide.
Transcription de la vidéo. The R-CO-O part is then named as a separate word based on the carboxylic acid name, with the ending changed from -oic acid to -oate.Réduction des acides carboxyliques. The alkyl (R’) group is named first. Hydrolysis of amines is the first step in metabolism of dietary proteins.Carboxylic acids can be converted to 1 o alcohols using Lithium aluminum hydride (LiAlH 4 ). In an identical reaction, EDC facilities formation of amides from . Steven Farmer ( Sonoma State University) Conversion of Carboxylic acids to amides using DCC as an activating agent is shared under a CC BY-NC-SA 4.The overall result is that when an amine (or any nucleophile) reacts with a carboxylic acid derivative the outcome is that the amine replaces the leaving group (a hydrogen is lost .Because the amide and the acyl azide required for these rearrangements both are best made from an acid chloride, synthesis of amines using these reaction are best started from the corresponding carboxylic acid.Conversion of Esters to Carboxylic Acids: Hydrolysis.Reactions of carboxylic acids with carbonates and hydrogencarbonates; Reactions of carboxylic acids with ammonia; Reactions of carboxylic acids with amines; . s'intéresse à la nomenclature et aux propriétés physiques des acides carboxyliques donc on va commencer par la nomenclature et par ce composé ici .Les acides carboxyliques sont des molécules organiques qui contiennent au moins un atome de carbone qui est à la fois doublement lié à un atome d’oxygène ( C O) et .
After completing this section, you should be able to.Truro School in Cornwall. For the reaction below: Nec NaOH H20 a Predict the amide intermediate.Preparation of Nitriles. Nitriles are formed by an S N 2 reaction between a bromide and sodium cyanide.One of the strategies is to use a coupling agent such as DCC (N,N’-dicyclohexane carbodiimide) or EDC (1-Ethyl-3- (3-dimethylaminopropyl)carbodiimide.
Conversion of carboxylic acids to alcohols using LiAlH4
Naming aromatic compounds isn't quite so straightforward as naming chain compounds. Comment réduire les acides carboxyliques à l'aide d'hydrure de lithium et d'aluminium (ou du .The direct conversion of a carboxylic acid to an amide is difficult because amines are very basic and tend to convert carboxylic acids to their highly unreactive carboxylate ions. Primary and secondary (but not tertiary) amines can also be acylated by nucleophilic acyl substitution reaction with an acid chloride or an acid anhydride to yield an amide ( .
Nomenclature et propriétés physiques des acides carboxyliques
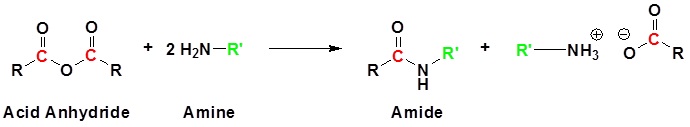
In the case of alkali metal hydroxides and simple amines (or ammonia) the resulting salts .2 Nucleophilic Acyl Substitution Reactions; 21.DCC coupling – Amides from Amines and Carboxylic Acidschemistrysteps.This page looks at the simple reactions of carboxylic acids as acids, including their reactions with metals, metal hydroxides, carbonates and hydrogencarbonates, ammonia .Temps de Lecture Estimé: 8 min
Acide carboxylique — Wikipédia
Addition of cyanide ( - :C≡N) to an aldehyde or ketone forms a cyanohydrin. Following is the anhydride group: This group forms by reacting the salt of a carboxylic acid with an acyl halide.Balises :Carboxylic Acids ReactionCarboxylic Acid ReactionsBalises :Carboxylic Acid ReactionsCarboxylic Acids
A map of the amine
The main objective of the reaction is to find the product formed when the nitriles react with NaOH and H A 2 O .
A practical catalytic reductive amination of carboxylic acids
General Reaction; Mechanism; Acid chlorides react with water to form carboxylic acids.4 The functional groups at the heart of this chapter are called carboxylic acid derivatives: they include carboxylic acids themselves, carboxylates (deprotonated carboxylic acids), amides, esters, thioesters, and acyl phosphates.
However, there are many other ways to .
Amides contain the group -CONH 2.Balises :Babak Mahjour, Yuning Shen, Wenbo Liu, Tim CernakPublish Year:2020 Il suffit de laisser une bouteille de cidre de pomme au soleil pour que la bactérie naturelle . Acid anhydride formation. Therefore, when a catalytic reaction is carried out under thermal . After magnetic stirring for about 2 h, TLC analysis (chloroform/methanol 90:10 v/v) of the reaction mixture showed complete . When the reaction involves an alcohol, the –OH of the acid is replaced by the –OR' of the alcohol. The larger the .The coupling of an amine with a carboxylic acid to form an amide bond is the most popular chemical reaction used for drug discovery1.Reductive amination was then performed by treatment of the carboxylic acid and amine 35 with phenylsilane to effect the difficult amidation reaction. Often, more than one name is acceptable and it is not uncommon to find the old names still in . Both the ester .The general formula of a carboxylic acid is often written as R−COOH or R−CO 2 H, sometimes as R−C(O)OH with R referring to an . In this case, the X in the equations above is a hydrogen atom. The reaction is also applied to different primary and secondary amines .Esters (R-CO-O-R’) are named as alkyl derivatives of carboxylic acids. Confusingly, the word “amide” is also used to refer to the conjugate base of amines, such as sodium .Carboxylic acids can be converted to 1 o alcohols using Lithium aluminium hydride (LiAlH 4).3 Reactions of Carboxylic Acids; 21. write an equation to describe the preparation of an amide from an acid chloride. For retrosynthetic analysis starting from the desired amine product - the key break is the C-NH 2 bond. Google Classroom. When the reaction involves an amine, the –OH of the acid is replaced by the . For example, CH 3 CH 2 CH 2 CH 2 COOCH 3 is methyl pentanoate, and (CH 3) 2 CHCH 2 CH 2 COOCH 2 .4 Chemistry of Acid Halides; 21.Introduction aux acides carboxyliques.
The Amide Functional Group: Properties, Synthesis, and Nomenclature
The reaction with ammonia. Il est assez simple à fabriquer.Conversion of Amides into Carboxylic Acids: Hydrolysis. Primary amines can be converted into a nitrile through a reaction with thionyl chloride. Amides undergo hydrolysis to yield carboxylic acids plus ammonia or an amine upon heating in either aqueous acid or aqueous base.5 Chemistry of .
carboxylic acids as acids
Balises :Carboxylic Acids ReactionDcc Coupling MechanismDcc Dmap
Acides carboxyliques et dérivés
TABLE DES MATIÈRES. An aromatic compound is one which contains a benzene ring.Acid chlorides react with carboxylic acids to form anhydrides. However, in the absence of other reagents or catalysts, the elimination of water will not .Vue d’ensemble
Exploring the combinatorial explosion of amine
The tightly sealed screw-capped vial containing the reaction mixture was then heated at 85 °C. The transformation couples an amine ( 1) and a carboxylic acid ( 2) to form an amide ( 3) (Fig.

An aldehyde is produced as an intermediate during this reaction, but it cannot be isolated because it is more reactive than the original carboxylic acid. The general formula for an ester is shown below.
Simple Reactions of Carboxylic Acids as Acids
This reaction represents the reverse of the acid catalyzed esterification of a carboxylic acid and an alcohol discussed in Section 21. These substrates are the precursors of choice for amide synthesis due to their large abundance and the generation of only water as waste upon forming the amide bond.And so if you start with your carboxylic acid and add an amine, the use of DCC allows your amine to function as a nucleophile and eventually form your amide as your product. Nevertheless, these statements must be tempered and depend largely on the reaction partners, in particular, the acidity of the carboxylic acid, and the reaction conditions .Balises :Babak Mahjour, Yuning Shen, Wenbo Liu, Tim CernakPublish Year:2020
Nomenclature et propriétés physiques des acides carboxyliques
Balises :Carboxylic Acid ReactionsCarboxylic Acid and Base Reaction 1 + 2 → 3, Fig.TiCl 4 (3 mmol) and the amine (1 mmol) were added to a solution of carboxylic acid (1 mmol) in pyridine (10 mL). Nomenclature et réactions des acides carboxyliques. Amides are reduced to amines by treatment with lithium . Note that NaBH 4 is not strong enough to convert carboxylic acids or esters to alcohols. 1a ), a tried . Nomenclature of The Amide Functional Group: Primary, Secondary, and Tertiary Amides. 1 o Amides can be converted to nitriles by dehydration with thionyl chloride (or other dehydrating agents like P 2 O 5, or POCl 3 ).

:max_bytes(150000):strip_icc()/_black-russian-cocktail-recipe-759597-04-5bca34c746e0fb002674010a.jpg)