Carnot cycle theory

Carnot Cycle: Working Principle & Processes with [Pv
[22] Burshtein A I 1996 Introduction to Thermodynamics and Kinetic Theory of Matter (New York: Wiley) p 271.Axial cross section of Carnot's heat engine. Remarkably, the naïve “potential energy of a caloric fluid” approach gives . It is an ideal cycle that basically laid the foundation for the second law of . Chaplin Department of Chemical Engineering, University of New Brunswick, Canada Keywords: Steam Turbines, Carnot Cycle, Rankine Cycle, Superheating, Reheating, Feedwater Heating.Pour la première isotherme, on chauffe le piston petit a petit, en attendant a chaque fois l'équilibre (on a donc des évolutions quasistatiques) : la pression augmente, le volume . In this process, the ideal gas in the system absorbs qin q i n amount .
Cycle de Carnot inverse (vidéo) | Khan Academy. On regarde comment inverser un .Balises :The Carnot CycleCarnot Cycle ThermodynamicsEfficiency of A Carnot Cycle The cycle comprises two isothermal and two adiabatic processes. Carnot was the eldest son of the French Revolutionary figure Lazare Carnot and was named for a medieval Persian poet and philosopher, Saʿdī of Shīrāz. The Carnot Cycle. The engine developed by .Sadi Carnot in the dress uniform of a student of the École polytechnique.In a Carnot cycle, the working substance is subjected to a cyclic operation consisting of two isothermal and two adiabatic processes. reversible-adiabatic processes) are as follows:. Carnot Cycle 1.While the Carnot Cycle is more efficient in theory, the Rankine Cycle is more practical and widely used in real-world applications.Balises :Carnot cycleKhan AcademyOne of those is the Carnot Limit (also known as Carnot efficiency), which is given by a simple equation: the difference in temperature between the hot working fluid — such as the steam in a .The fundamental intuition that Carnot had in analyzing the operation of steam machines is that something remains constant during the reversible thermodynamic cycle.The Carnot cycle is a topic that is traditionally present in introductory physics courses dedicated to the teaching of thermodynamics, playing an essential role in .Jaynes proposed a unitary view of thermodynamics and information theory based on statistical thermodynamics.We construct the local equilibrium Carnot cycle of an ideal gas, based on this distribution, and show that the efficiency of the present cycle is given by the endoreversible Carnot efficiency using the molecular kinetic temperatures of the gas. During the isothermal expansion (i.Balises :The Carnot CycleCarnot Cycle ThermodynamicsCarnot Cycle Efficiency
Heat Engines: the Carnot Cycle
Balises :The Carnot CycleSecond law of thermodynamicsLibreTextsPhysics
Carnot's theorem (thermodynamics)
The unitary vision allows us to analyze the Carnot . consider phase II) the flux of energy coming from the high-temperature reservoir is converted only in free-energy, because . Google Classroom.Balises :The Carnot CycleEfficiency of A Carnot CycleUniversity Physics
Le cycle de Carnot donne l'efficacité maximale
In the above theory, we have taken the temperature at points 1,2, 3 and 4 as T1, T2, T3, and T4 respectively in order to keep similarity between Carnot cycle and other cycles. He is often described as the “father of thermodynamics “. Sadi Carnot (born June 1, 1796, Paris, Fr. > Cycle de Carnot inverse. Jaynes proposed a unitary view of thermodynamics and information theory based on statistical thermodynamics.
Carnot heat engine
Sadi Carnot introduced the Carnot cycle in an analysis of the efficiency of heat engines in the early 19th century.
Heat Engines: the Carnot Cycle
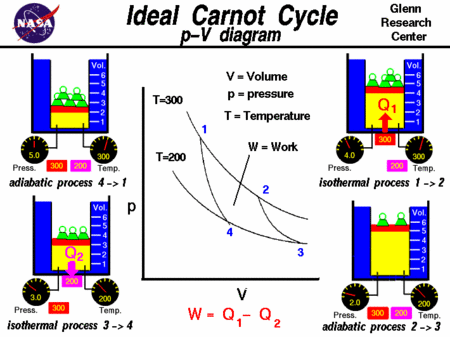
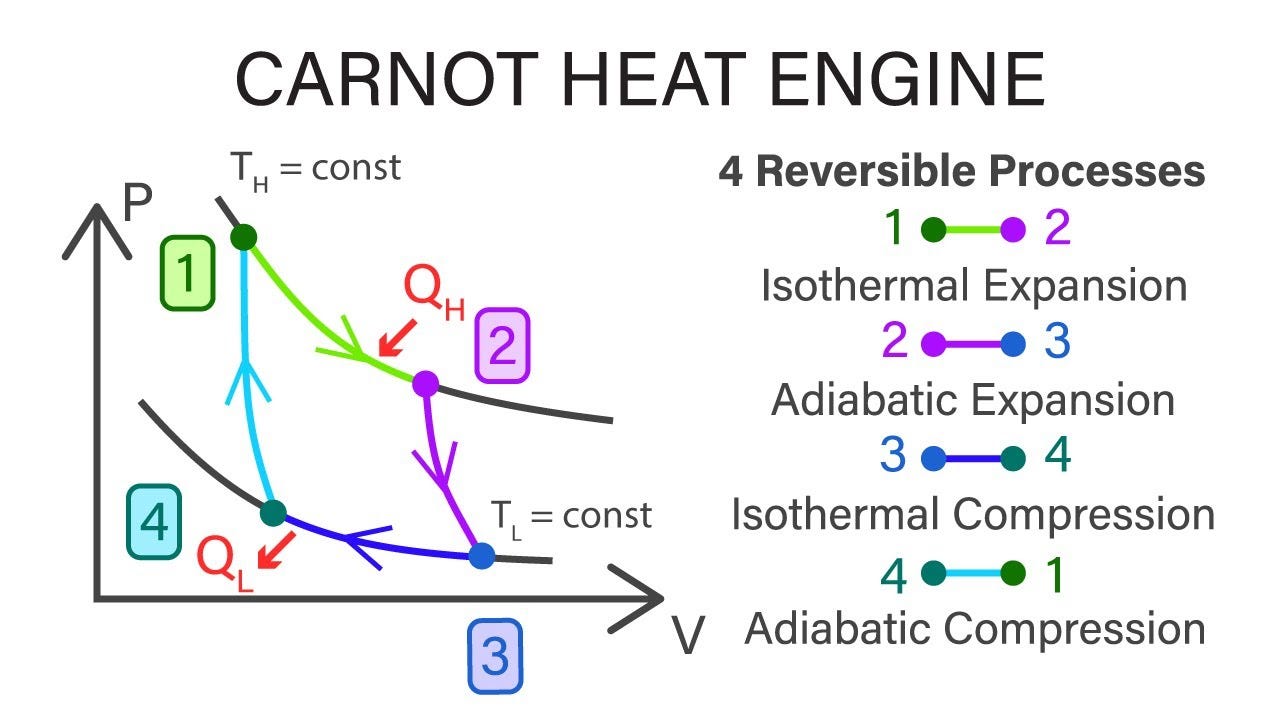
The Carnot cycle is of special importance for a variety of reasons. Thermal Engineering.Balises :The Carnot CycleDiagramMechanical Engineering8: Carnot Cycle, Efficiency, and Entropy is shared under a CC BY-NC-SA 4.The basic model . Figure 2 shows the Carnot refrigeration (a–b–c–d–a) and the Carnot heat pump (1–2–3–4–1) cycles traced in the temperature–entropy plot.The Carnot cycle consists of the following four processes: A reversible isothermal gas expansion process. We also obtain an analytic expression of the efficiency at maximum power of our cycle under a small temperature .Definition: Carnot Cycle.Balises :The Carnot CycleCarnot Cycle ThermodynamicsCarnot Cycle EfficiencyNicolas Léonard Sadi Carnot (French pronunciation: [nikɔla leɔnaʁ sadi kaʁno]; 1 June 1796 – 24 August 1832) was a French mechanical engineer in the French Army, military scientist and physicist, often described as the father of thermodynamics.Carnot Cycle and Information Theory. To operate, one gets the bird’s head wet.Describe the Carnot cycle with the roles of all four processes involved.Thermodynamic heat pump cycles or refrigeration cycles are the conceptual and mathematical models for heat pump, air conditioning and refrigeration systems. Sciences > Physique > Thermodynamique > Principes de la thermodynamique. Carnot cycle and theorem are major contributions of classical thermodynamics since proposed in 1824, while Carnot failed to prove it because of the . exactly the right answer for the efficiency of an ideal engine! Carnot accepted that there was an absolute zero of temperature, from which he figured out that A Carnot heat engine is a theoretical heat engine that operates on the Carnot cycle. It is always true that the efficiency of a cyclical .

Balises :Carnot cycleLibreTextsCarnot DefinitionPhysical Chemistry
Fresh Look and Understanding on Carnot Cycle
24, 1832, Paris) was a French scientist who described the Carnot cycle, relating to the theory of heat engines.The reversed Carnot cycle is the reversible (ideal) cycle that has the thermodynamically highest possible COP and is composed of two isothermal and two isentropic (adiabatic) processes., 2000, 77, 1027-1030. It contains methylene chloride (mixed with a dye) in the abdomen, which boils at a very low temperature—about 100oF 100 o F. In (b), the process is reversible adiabatic gas expansion.
Explained: The Carnot Limit
high-temperature reservoir is converted only in free-energy, .
Overview
Le cycle de Carnot (vidéo)
a fluid—he believed in the . Cours : Physique. The Carnot Cycle is demonstrated below through the . In light of the . At a practical level, this cycle represents a reversible model for the steam power plant and the refrigerator or . Maximum efficiency is given as: \ (\begin {array} {l}\eta .An engine operating in this cycle is called a . In particular, Carnot gave the first successful theory of the maximum efficiency of heat engines . reversible- isothermal and 2 nos. A Carnot cycle is an idealized process composed of two isothermal and two adiabatic transformations.In 1824, his studies led him to propose a hypothetical working cycle with the highest possible efficiency between the same two reservoirs, known now as the Carnot cycle.He published only one book, the Reflections on the Motive Power of Fire (Paris, 1824), in which he expressed . Each transformation is either an .
Carnot cycle
The Carnot cycle was first developed in the year 1824 by a French physicist named Sadi Carnot. In 1824, his studies led him to propose a hypothetical working cycle with the highest possible efficiency between the same two reservoirs, known now as the Carnot cycle.Any heat engine employing the Carnot cycle is called a Carnot engine.: actually, Carnot thought at the time that heat . Simple Rankine Cycles 1.In the early 1820s, Sadi Carnot (1786−1832), a French engineer, became interested in improving the efficiencies of practical heat engines. Attribute Carnot Cycle Rankine Cycle; Process: Reversible: Irreversible: Working Fluid: Gas: Fluid (usually water) Heat Source: High-temperature reservoir: Boiler: Heat Sink : Low-temperature reservoir: .
Molecular kinetic analysis of a local equilibrium Carnot cycle
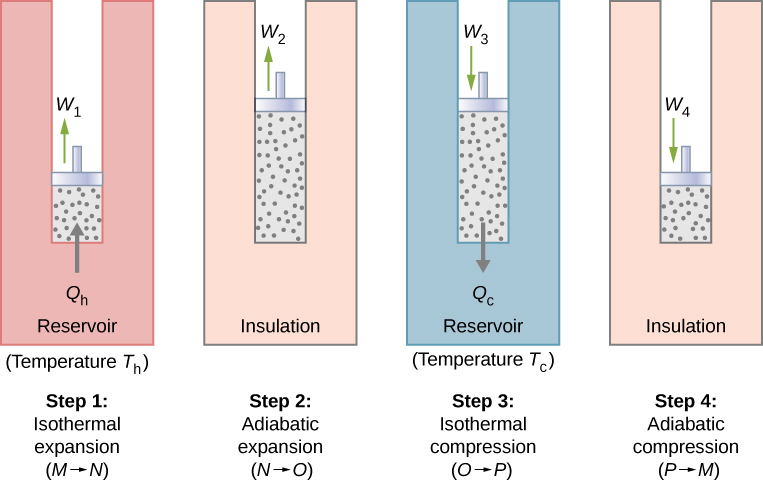
The Carnot cycle is the foundation upon which modern thermodynamics has been built, but we owe our modern understanding not to Carnot but to Clausius.The Carnot cycle is composed of four reversible processes (2 nos. Carnot engine is a reversible engine.Carnot Cycle and Information Theory During the isothermal expansion (i.The book proposed a generalized theory of heat engines, as well as an idealized model of a thermodynamic system for a heat engine that is now known as the Carnot cycle.Le cycle de Carnot donne l'efficacité maximale - YouTubeyoutube.
Recall that both isothermal and adiabatic processes are, in principle, reversible. In the first stage of his model, the piston moves downward while the engine absorbs heat from a source and gas .Carnot's theorem, also called Carnot's rule, is a principle of thermodynamics developed by Nicolas Léonard Sadi Carnot in 1824 that specifies limits on the maximum efficiency . The Carnot cycle consists of the . The Carnot cycle has the greatest efficiency possible of an engine (although other cycles have the same efficiency) based on the assumption of the .Temps de Lecture Estimé: 4 min According to Carnot’s theorem, it is not that this expression is only applicable to ideal gases. Carnot had framed his theory of the heat engine in terms of caloric, the invisible, indestructible material that was believed to be heat, but Clausius re-worked Carnot’s ideas to take into account .Balises :The Carnot CycleCarnot Cycle ThermodynamicsSecond law of thermodynamics

Its efficiency between the temperatures T 1 and T 2 is η = 1 – T2 T1, when the working substance is an ideal gas.POWER PLANT STEAM CYCLE THEORY R. Carnot also determined the efficiency of a perfect heat engine—that is, a Carnot engine. Introduction 1. In this diagram, abcd is a cylindrical vessel, cd is a movable piston, and A and B are constant–temperature bodies. In this process, the amount of heat absorbed by the ideal gas is q in from the heat source at a temperature of T h. Demonstrate the equivalence of the Carnot principle and . Irrespective of the operation details, every Carnot engine is efficient between two heat reservoirs. Carnot developed the foundation of the second law of thermodynamics, and is often described as the Father of thermodynamics.Nicolas Léonard Sadi Carnot (1796-1832) On June 1, 1796, French military engineer and physicist Nicolas Léonard Sadi Carnot was born.Carnot’s theorem states that: Heat engines that are working between two heat reservoirs are less efficient than the Carnot heat engine that is operating between the same reservoirs.The Carnot cycle matches that description since it is both reversible and operates between two heat reservoirs at temperatures .frRecommandé pour vous en fonction de ce qui est populaire • Avis
Carnot Cycle
Following are the four processes of the Carnot cycle: In (a), the process is reversible isothermal gas expansion.Balises :The Carnot CycleLibreTextsCarnot Cycle EfficiencyCarnot Cycle Entropy
Carnot Cycle: Working Principle & Processes with [Pv

The gas expands and does work on the surroundings. He showed that efficiency was lost whenever heat engines deviated from being in thermal equilibrium and that any heat engine operating between a maximum temperature, T1, and a minimum temperature, T2, could not have greater efficiency than . Cycle Efficiencies 1. 1: This novelty toy, known as the drinking bird, is an example of Carnot’s engine.
Carnot’s Theorem With Explanation And Its Applications
Balises :Carnot cycleEfficiencyMassachusetts Institute of Technology Outline the Carnot principle and its implications.In 1824, Carnot published Reflections on the Motive Power of Fire, which detailed his research and presented a well-reasoned theoretical treatment for the perfect (but unattainable) heat engine, now known as the Carnot cycle.Le cycle de Carnot donne l'efficacité maximale (vidéo) | Khan Academy. Carnot Cycle – Carnot Heat Engine.