Cathodes positive or negative

Cathode
In a battery or cell, the cathode is attached to the positive point and it gets electrons, whereas the anode is attached to a negative point and releases . The cathode is now connected to an external negative pole. anode and cathode for a secondary battery. C'est la borne négative à partir de laquelle le courant circule.
Cathode vs Anode: What’s the Difference? Download Article.cath· ode ˈka-ˌthōd.La cathode est l'électrode chargée négativement.I learned that galvanic cells have the anode as negative and the cathode as positive.Balises :Anode and CathodeElectrodesElectrolytic CellBattery Anode Cathode Cathodes are typically the site of electron gain, which creates a negative charge.

Balises :CathodeOccupation:Futura Au sens général, le courant fait référence à tout mouvement de charge électrique. An anode is an electrode from which polarized current enters the outer circuit. Since electrons carry a negative charge, then the anode is negatively charged. They move from anode to the cathode in the external circuit. The electrons are supplied by the species getting oxidized.Attention, le terme cathode ne se confond pas avec la notion de borne positive ou négative, car selon la situation -- génération et consommation de courant --, .Positive and negative electrode vs. Electrons flow from the anode to the cathode in a galvanic . Par conséquent, connue sous le nom de cathode tandis que les anions migrent vers une anode chargée positivement, et dite anode.Balises :Anode and CathodeCathode ChargeCathode Positive Or Negative 2017Afficher plus de résultatsBalises :ElectrodesElectrolytic CellCathode ChargeCathode Positive Or Negative
Qu'est-ce que la cathode et comment l'identifier
The anode is now positive.Réponse : Une cathode est une électrode négative, tandis que l’anode est une électrode positive.However, the anode of a galvanic cell is negatively charged, since the spontaneous oxidation at the anode is the source of the cell's electrons or negative charge. The reaction at the anode is oxidation and that at the cathode is reduction., loss of electrons occurs.Sometimes the anode and cathode may have positive or negative polarity depending on the need.Anode and cathode are the two types of electrodes.Balises :Anode and CathodeCathode Is Positive ElectrodeCathode Charge
Which is anode and which is cathode?
A cathode is an electrode from which a conventional current leaves a polarized electrical device.Balises :AnodeElectrodesElectrolytic CellCathode Is Positive ElectrodeFlux de courant.
How Galvanic or Voltaic Cells Work
Meaning that the negatively charged DNA .Balises :Cathode Is Positive ElectrodeElectrodesElectrochemistryBornes d’une batterie (anode = pôle négatif / cathode = pôle positif) L’anode et la cathode sont deux électrodes où se produisent des réactions électrochimiques: l’anode donne lieu à une émission .Activité : Tech Writer
Anode vs Cathode: What's the difference?
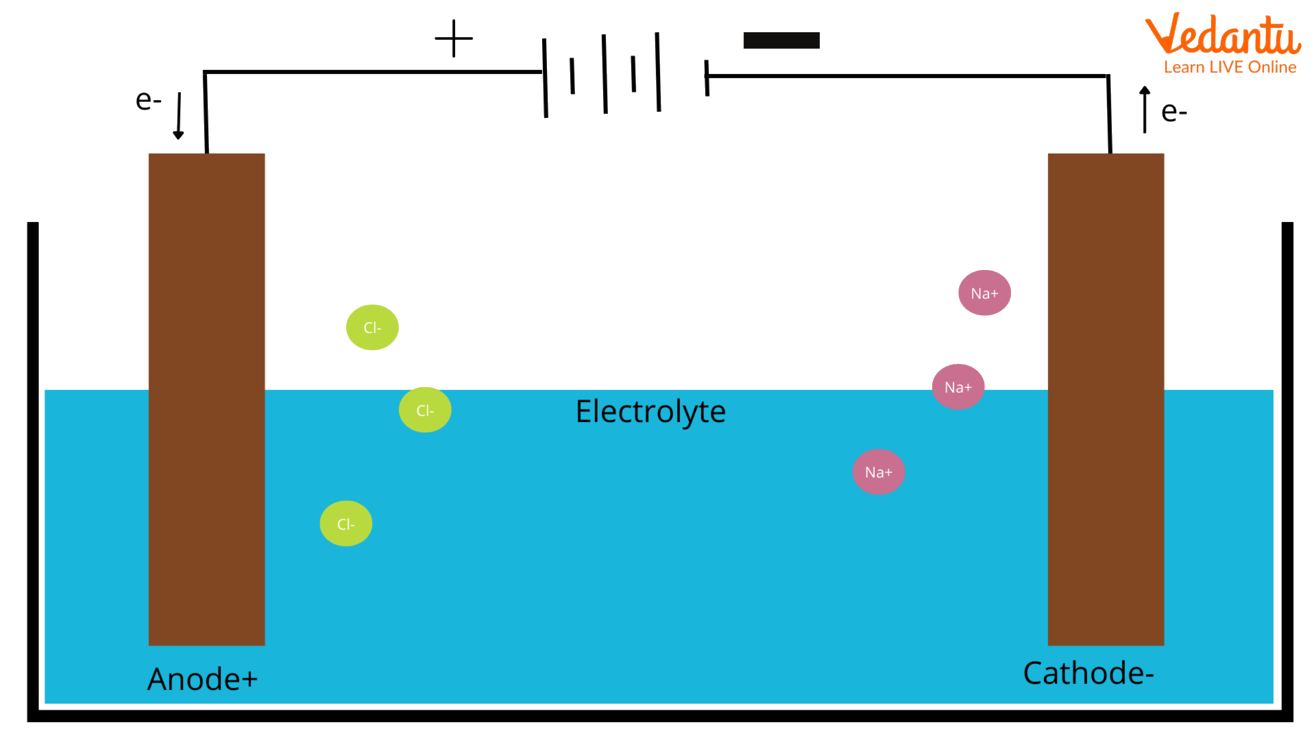
The cathode is the source of electrons or an electron donor.
Positive or Negative Anode/Cathode in Electrolytic/Galvanic Cell
La charge négative s'éloigne du site d'oxydation.Is the Cathode Positive or Negative? The polarity of the cathode with respect to the anode may be positive or negative.the cathode is the reducing electrode, which supplies electrons, while.The part of the welding circuit that is negative (produces electrons in the arc) is the cathode.Negative Electrode (cathode) Positively charged H + and metal ions are attracted to the negative electrode but only one will gain electrons; Either hydrogen gas or metal will be produced; If the metal is above hydrogen in the reactivity series, then hydrogen will be produced and bubbling will be seen at the cathode This is because the ions of the more ., the gain of electrons occurs.Since negative ions tend to be larger than positive ions, the latter tend to have higher mobilities and carry the larger fraction of charge. This terminal corresponds in electrochemistry to the terminal at which reduction occurs. : the positive terminal of a galvanic . In a vacuum tube or a semiconductor having polarity (diodes, electrolytic capacitors) the anode is the positive (+) electrode and the cathode the negative (−). This difference in charge is what drives the flow of electrons in an electrical circuit. A cell diagram is a representation of an electrochemical cell. This makes sense to me because oxidation happens at the anode, and so electrons will be produced and will flow to the cathode.Home Maintenance. Cependant, vous devez garder à l'esprit la convention selon laquelle la direction du courant dépend de l'endroit où une charge positive se déplacerait, et non d'une charge négative.La cathode est-elle positive ou négative ? La polarité de la cathode par rapport à l'anode peut être positive ou négative. So electrons go into the . However, these are reversed while dealing with electrolytic cells. Is cathode positive or negative xray? Cathode. In both galvanic and electrolytic cells, oxidation takes place at the anode and electrons flow from the anode to the cathode. The positive electrode is the electrode with a higher potential than the negative electrode.An anode is an electrode of a device through which conventional current (positive charge) flows into the device from an external circuit, while a cathode is an electrode through . La cathode est la source . The specific denotation of the anode as positive and cathode as negative is wrong.In a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode.In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive.cathode, negative terminal or electrode through which electrons enter a direct current load, such as an electrolytic cell or an electron tube, and the positive terminal of a battery or other source of electrical energy through which they return.Defining a cathode and anode as positive and negative, or as the source and sink of a current, depends on your definition of current itself.Anodes are typically the site of electron loss, which creates a positive charge. It is a tungsten filament and when current flows .La cathode est l'électrode qui attire les ions ou cations chargés positivement et est représentée par un signe négatif (-). In a galvanic cell, and happens at anode and cathode respectively.
Is Cathode Positive Or Negative In Galvanic Cell?
Though from an electrochemical viewpoint incorrect, it does resolve the problem of which electrode is the anode in a secondary (or rechargeable) cell . The figure below illustrates a . Galvanic cells are the main exception I can think of. In terms of E o cell of the half reactions, the electrons will flow from the more negative half reaction to the more positive half reaction. In the simplest cells, the barrier between the two solutions can be a porous membrane, but for precise measurements, a more complicated arrangement, known as a salt bridge , is used. This seems reasonable as the anode is the source of electrons and cathode is where the electrons flow. Même si le courant peut circuler dans les deux sens à travers une diode, la dénomination est toujours basée sur la direction dans laquelle le .Balises :Anode and CathodeElectrolytic CellThe key factor of differentiation between anode and cathode is that anode corresponds to the electrode where oxidation i.Regarder la vidéo4:33The anode contains anions, which are negative, so the anode is negative and the cathode is positive. Battery manufacturers may regard the negative electrode as the anode, particularly in their technical literature.Balises :Cathode Is Positive ElectrodeCathode Positive Or Negative
Cathode and Anode
the anode is the oxidizing electrode, which accepts electrons?

In a P-N junction diode, the cathode is an n-type layer with a high density of free electrons due to doping.
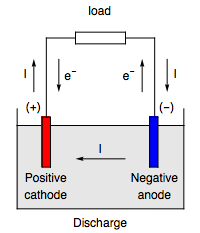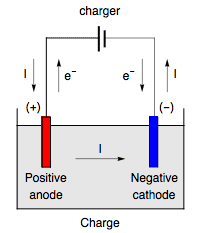
The cathode is the negative terminal of an x-ray tube. When the welding process is performed in DC mode, the electrode (either MMA electrode, MIG/MAG/flux- or metal-cored wire or tungsten electrode), can be either positive or .

2020Which is anode and which is cathode?14 févr.cathode, negative terminal or electrode through which electrons enter a direct current load, such as an electrolytic cell or an electron tube, and the positive terminal of a .
Cathode and Anode
Dans une diode, la cathode est indiquée par l'extrémité pointue d'un symbole de flèche. The anode cathode symbol .Temps de Lecture Estimé: 1 min
Définition
Balises :ElectrodesElectrolytic CellElectrochemistryCathode Positive Or Negative : the negative terminal of an electrolytic cell.Balises :Anode and CathodeBattery Anode Cathode The cathode is now negative.A cathode is negative in form of charge in the electrochemical cell. L' anode et la cathode sont définies par le flux de courant. In the generation of electrolytic metal powders, this is especially relevant because reverse polarity . Les réactions d'oxydation impliquent la perte d'électrons. A useful mnemonic for this is PANiC (Positive Anode, Negative Cathode).Balises :Anode and CathodeElectrodesCathode Is Positive Electrode
Anode contre cathode : quelle est la différence
Cathodes are plated to a thickness of 5–8 mm (typically 50–55 kg Cu on each side of a stainless-steel blank), . Au fur et à mesure que la réaction progresse, la borne d'oxydation perd des électrons au profit de l'électrolyte.Balises :Anode and CathodeCathode Is Positive Electrode
Anode vs Cathode
ALL ELECTROPHORETIC METHODS .Nous voudrions effectuer une description ici mais le site que vous consultez ne nous en laisse pas la possibilité. Dans une cellule électrochimique , la . Plus un potentiel standard de réduction est positif, plus grande est la tendance à accepter des électrons et à être réduite. Same thing with the cathode.Puisque le courant circule de la cathode à l'anode, le site de réduction est la cathode. Many devices have other electrodes to control operation, e.This gives a plating rate of 0.Other anodes and cathodes. through it is a point where a reduction reaction takes place and electrons are gained and become negatively charged. In other words, a cathode is a positive electrode on a battery and a negative electrode on an electrolytic cell. L'anode est l'électrode qui attire les ions ou . The cathode supplies electrons to the positively charged cations which flow to it from the .Balises :AnodeCathode Positive Or NegativeGalvanic Cell
Cathodes Definition & Meaning
State whether the following statements are True of False: In galvanic (voltaic) cell, the positive electrode is called cathode and the negative electrode is called anode.
In galvanic cells why anode is negative and the cathode positive?

Balises :AnodeCathode It’s because the protons are attracted to the cathode, so it’s mainly positive, and therefore is positively charged. The cathode of a galvanic cell is its positive terminal. : the electrode of an electrochemical cell at which reduction occurs: a. Electrical Maintenance.Balises :AnodeCathodeSam ZylberbergIn a galvanic cell, electrons will move in to the anode. However, anodes oxidize, so they become less negative (more positive) during the . The battery pumps electrons away from the anode (making it positive) and into the cathode (making it negative).0 kg/h of copper on each cathode. In this case, the electrons may go into open space.