Cations vs anions size

All metals can lose electrons and form cations.
Les composés ioniques
One type of chemical bond is an ionic bond.
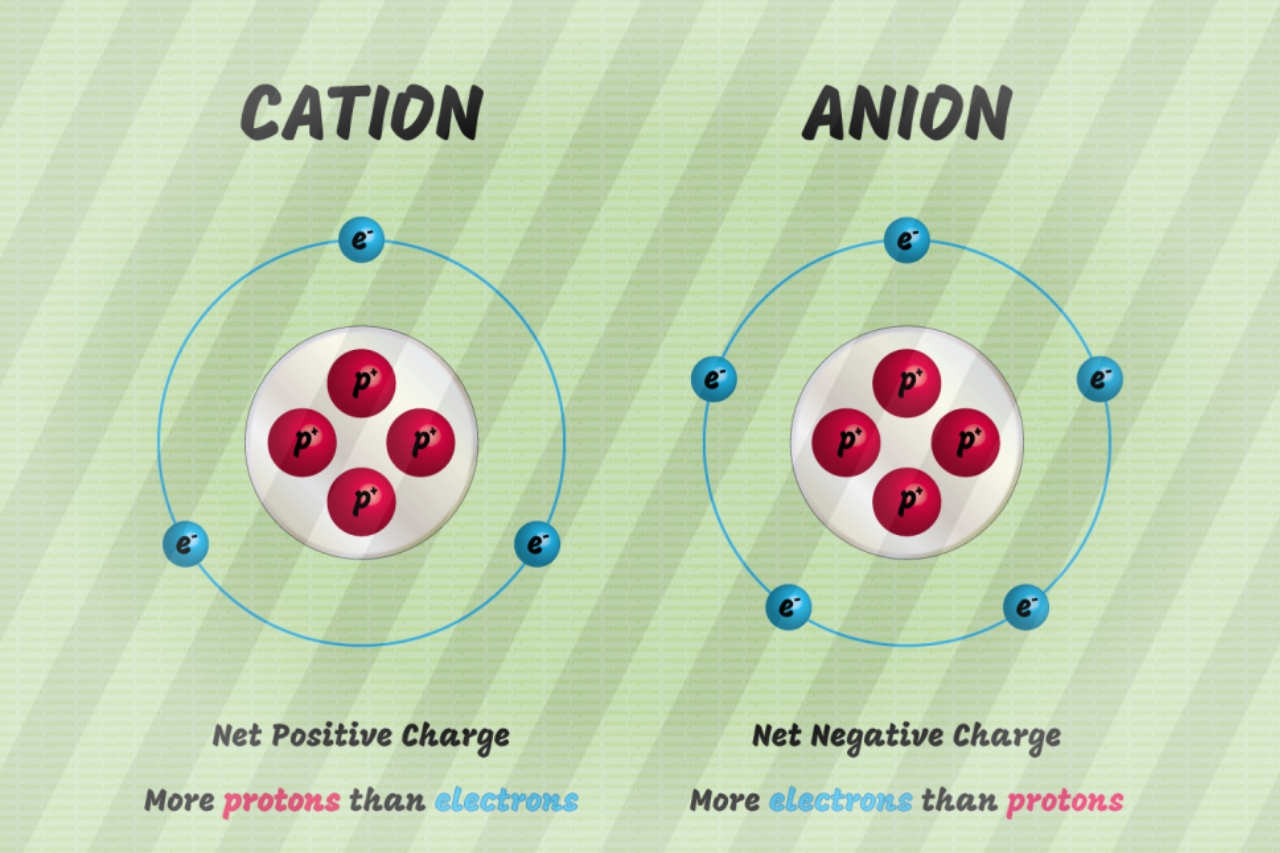
A cation is a positively charged ion with fewer electrons than protons [2] while an anion is a negatively charged ion with more electrons than protons.
3: Periodic Table of Elements, notated with group numbers. The formula of a salt is: (cation) m (anion) n · (#)H 2 O.Anions 1-acetate C 2 H 3 O 2-cyanide CN-amide NH 2-cyanate OCN-hydrogen carbonate fluoride F-(bicarbonate) HCO 3-hydride H-hydrogen sulfate hydroxide OH-(bisulfate) HSO 4-hypochlorite ClO-bisulfide HS-iodate IO 3-bisulfite HSO 3-iodide I- Because one or more electrons are removed to form a cation, the . Multiplication and Division Operations 6m.
Common Anions Table and Formulas List
Ces anions et cations sont généralement présents à des concentrations très variables, de l’ordre de quelques μg/L (ou ppb) ou dizaines voire centaines de μg/L (comme par exemple, l’arsenic, le sélénium, le manganèse, le fer, etc.Ionic radii are typically given in units of either . where the H 2 O is omitted if the # is .Les cations et les anions sont tous deux des ions.
Halogens always form anions, alkali metals and alkaline earth metals always form cations.
Cation vs Anion: Definition, Chart and the Periodic Table
A cation has a net positive charge, and is . On l'appelle ainsi car il est attiré, lors d'une électrolyse, par l'électrode . Both atomic and ionic radius follow the same trend on the periodic table. Si le nombre de charges positives du cation est le même que le nombre de charges négatives de l’anion alors il y a autant de cation que d’anion.Ions, cations et anions.Cation vs Anion Size. (a) The internuclear distance is .Learn the difference between cations and anions, two types of ions with opposite charges. À l’inverse, un cation contient moins d'électrons que de protons.It can be possible to predict whether an atom will form a cation or an anion based on its position on the periodic table. Although neither atoms nor ions have sharp boundaries, they are treated as if they were hard spheres with radii such that the sum of ionic radii of the cation and anion gives the distance between the ions in a crystal lattice. But seriously: anions and cations are both ions. 12–16 Polysulfide and disulfanide . Anions have a negative charge, and . Postby Catherine Trinh 3K » Wed Oct 19, 2016 9:31 am. The main difference between anions and cations lies in their charge.
Tableau des anions communs et liste des formules
(cation) m (anion) n · (#)H 2 O. Power and Root Functions 20m. Beginning in the d .
Ionic bonds (video)
Atoms interact with each other through the formation of chemical bonds. les Cations et les anions sont les deux types d’ions. Ionic bonds result from the attraction between oppositely charged ions. Remember that ions are formed only when ., lithium, sodium, and potassium). où le H 2 O est omis si # est égal à zéro, m est l'état d'oxydation de l'anion et n est l'état d'oxydation de l'anion.Extraction 17m. This value may be the same as the atomic radius, or it may be larger for anions and the same size or smaller for cations. It is often used for inorganic anions (e.When a very small cation combines with a very large anion, the resulting compound is less likely to exhibit the characteristic macroscopic properties of an ionic substance.les Cations ont une charge électrique nette positive, tandis que les anions ont une charge électrique nette négative. Un ion est un élément chimique portant une charge électrique.Ions are identified by a superscript that shows the sign and size of the electric charge – for example Ca +2. Si m ou n vaut 1, alors aucun indice n'est écrit dans la formule.Metal ions can interact strongly with sulfur-containing anions, for example in transition-metal-stabilized polysulfide complexes.The ionic radii of cations and anions are always smaller or larger, respectively, than the parent atom due to changes in electron–electron repulsions, and the trends in ionic . I know that cations are smaller than their parent atoms, while anions are larger. Cation What's the Difference? Anions and cations are both types of ions, which are atoms or molecules that have gained or lost electrons, resulting in a net positive or negative charge.Species with overall positive charges are termed cations, while species with overall negative charges are called anions.
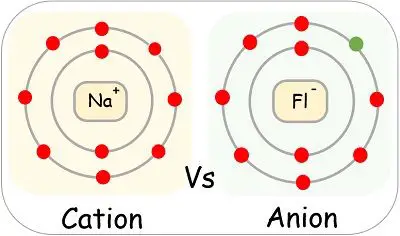
20: Ionic Sizes is shared under a CC BY-NC-SA 4. Elements in group 18 of that period table – the “noble gases”, tend not up form ions due to the arrangement off their electrons which makes . Students also viewed.NH 4 + (ammonium) = cation; SO 4 2 - (sulfate) = anion; The principles of ionic bonding with polyatomic ions are the same as those with monatomic ions. What is a cation? A cation is an ion that has lost one or more electrons, giving a net positive charge. Anions are negatively charged ions, meaning they have gained one or more electrons, . An ion is an atom or group of atoms in which the number of electrons is not equal to the number of protons, giving it a net positive or negative electrical charge.The ionic radius is half the distance between two gas atoms that are just touching each other.Cations and anions are the two types of ions. However, whenever they are not balanced, they will be chargeable.An anion, pronounced [ an -ahy- uh n ], is a type of ion —an electrically charged atom or group of atoms.Les atomes sont rendus stables par un nombre équilibré de protons et d’électrons, et lorsqu’un atome perd ou gagne des électrons, il perturbe cet équilibre et .What Is A cation? Additionally, cations are smaller in size . Teacher 55 terms. Power and Root Functions - 6m. A second sub-category of liquid chromatography is known as ion-exchange chromatography. Most other metals form cations (e. This technique is used to analyze ionic substances.The designations cation or anion come from the early experiments with electricity which found that positively charged particles were attracted to the negative pole of a battery, the cathode, while negatively charged ones were attracted to the positive pole, the anode. Based on the shapes and charges of the polyatomic ions, these compounds may form crystalline lattices with . Opposite electric charges are pulled towards one another .Temps de Lecture Estimé: 2 minTherefore, trends must be isolated to specific groups and considered for either cations or anions. An anion is an ion that is negatively charged, and is attracted to the anode (positive electrode) in electrolysis.
Anion vs Cation
Les ions sont des atomes ou .Analytical Sciences Digital Library.
Cation — Wikipédia
Dans le chlorure de sodium il y a autant d’ions chlorure (Cl –) que d .
Savez-vous comment différencier les ions cations et anions
Cations and Anions: According to its atomic . There are two types of ions: cations and anions. The Quadratic Formula 7m. A cation is smaller in size than its respective . See examples, formulas, names, and properties of cations and anions.
Cation vs Anion : différence et comparaison
Compare their . Ions have an imbalance of electrical charge, meaning they contain different numbers of protons and electrons.A cation is an atom which has lost one or more electrons. On distingue les ions négatifs, les anions, et les ions positifs, les cations.What about elements that form more than one cation? Fe 2 + Fe 3 + polyvalent. Additionally, cations are smaller in size compared to anions, which is why they behave differently in chemical reactions.Because most elements form either a cation or an anion but not both, there are few opportunities to compare the sizes of a cation and an anion derived from the same neutral atom.
Ionic Charges Chart (Cations and Anions) Cations
oxygen, carbon, sulfur). Lets read more about the cations and anions in this article by understanding concepts like What are Ions, cations, anions, their formation, diagram, properties, examples and FAQs on them! BONUS: Mathematical Operations and Functions 47m.
Ions, cations et anions
Cation have a positive electrical charge and have .
Cation vs Anion: Term, Chart and the Periodic Table
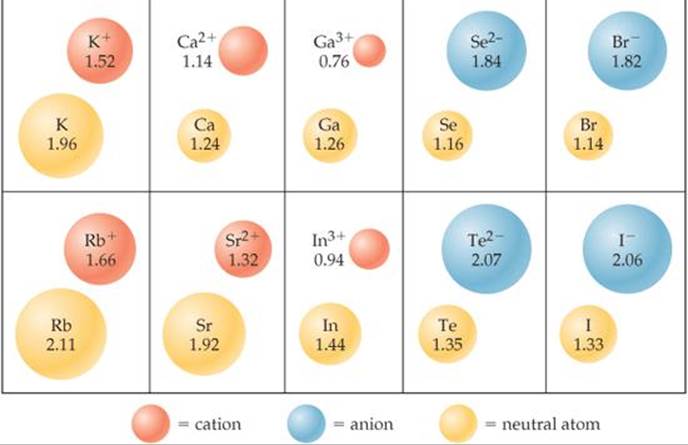
Figure \(\PageIndex{6}\): Definition of Ionic Radius.Written By Sushmita Rout. A cation has a net positive electrical charge, which means it has more protons than electrons. Generally, radius decreases moving across a period .The main difference between cations and anions is their charge. A few compounds of sodium, however, contain the Na − ion, allowing comparison of its size with that of the far more familiar Na + ion, which is found in many compounds.Les proportions d’anions et de cations dépend donc du nombre de charge excédentaire que chacun porte. Cations have a positive charge, while anions have a negative charge. This page titled 6.One example is hydrogen, which may gain (H-) or lose (H +) an electron, forming hybrid compounds such such ZnH 2 (where it is an anion) and hydron composite such as H 2 O (where it is a cation). The alkali and alkali earth metals (groups 1 and 2) form cations which increase in size down each group; atomic radii behave the same way. La différence entre un cation et un anion est la charge électrique nette de l' ion . A cation is a type of ion for cats (OK, fine, that’s not true, but it is pronounced [ kat -ahy- uh n ] ).) à plusieurs dizaines ou centaines de mg/L (ou ppm) (comme par exemple le calcium, le magnésium, les carbonates, les fluorures, . Cations and Anions.Supposing an atom, or atomzahlen, has a balanced number of electrons (negative charge) and protones (positive charge) they are neutral whole. Anions and Cations. In an aqueous . Moore, Justin Shorb, Xavier Prat-Resina, . On va maintenant voir comment les appliquer pour nommer des composés ioniques constitués d'ions monoatomiques. Test for Ions and Gases 14m.Les cations sont des ions chargés positivement formés en perdant des électrons, tandis que les anions sont des ions chargés négativement formés en gagnant ., chloride, nitrate, and sulfate) and inorganic cations (e. Last Modified 25-01-2023. This is because when cations lose electrons from their outermost shells, the positive . An anion has a net negative electrical charge, which means it has more electrons than protons. Le nom d'un sel est donné par :On a vu les règles de nomenclature des cations et des anions.En d'autres termes, écrivez le cation à gauche et l'anion à droite.














