Ch2 molecular formula

- Socratic27 nov. This program determines both empirical and molecular formulas.
Définition
Get control of 2022! Track your food intake, exercise, sleep and meditation for free. An empirical formula tells us the relative ratios of different atoms in a compound.1: If you count the carbons and hydrogens, you will see that they no longer fit the general formula CnH2n+2 C n H 2 n + 2. Count The Number of Each Atom. It will calculate the total mass along with the elemental composition and mass of each element in the compound. ∴ The molecular formula = CnH 2nOn = C₆H₁₂O₆. Calculamos pues n: n = peso molecular / peso fórmula mínima.CH2 (Methylene) molar mass.Molecular Formula.When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance.comStructure of methylene | Accounts of Chemical Researchpubs. Empirical formulas show the simplest whole-number ratio of atoms in a compound, molecular formulas show the number of each type of atom in a molecule, and structural formulas show how the atoms in a molecule are bonded to each other. Multiply each of the moles by the smallest whole number that will convert each into a whole number.12 g/mol and an empirical formula of C 2 H 5; determine the molecular formula for this compound. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.0 mole of oxygen. Los elementos se ingresarán utilizando su símbolo y se podrán agregar hasta . Use uppercase for the first character in the element and lowercase for the second character.1 are shown as condensed structures. For example, butane (C 4 H 10) has two possible structures. In many cases, the molecular formula is the .The molecular formula of a hydrocarbon provides information about the possible structural types it may represent. 1: Molecular Formulas. Examples: Fe, Au, Co, Br, C, O, N, F. For example, consider compounds having the formula \(\ce{C5H8}\). Enter an optional molar mass to find the molecular formula.comRecommandé pour vous en fonction de ce qui est populaire • Avis
Methylene
CH 2 Lewis structure. The smallest cycloalkane is cyclopropane. Benzene is an intermediate in the production of many important chemicals used in the manufacture of plastics, drugs, dyes, detergents and insecticides. Molecular Weight. The carbon atoms can form a single unbranched chain, or the primary chain of carbon atoms can have one or more shorter chains that form branches. This widget gets the Lewis structure of chemical compounds. We know that the molecular mass of the .Con esta calculadora conociento la composición centesimal de un compuesto podrás determinar la formula empirica y molecular.Molecular Formulas. The molecular formula tells the symbols of the elements that compose the compound, and the subscript to the element symbol denotes how many atoms of that element are in the molecule.Methylene (CH2): Structure, Properties and Uses - . Our job is to determine the value of n.In a molecular formula, it states the total number of atoms of each element in a molecule.
Molecular weight of CH2
The bonding in water results from overlap of two of the four sp3 hybrid orbitals on oxygen with 1 s orbitals on the two hydrogen atoms.The chemical formula for carbon dioxide is CO 2. 1: The empirical formula of a compound can be derived from the masses of all elements in the sample. The information on this page is fact-checked.orgRecommandé pour vous en fonction de ce qui est populaire • Avis
Methylene (CH2)
November 13, 2023 by Deep. This difference suggests such compounds may have .
Ethylene
3%) of the compound.Molecular formula.A molecular formula is a representation of a molecule that uses chemical symbols to indicate the types of atoms followed by subscripts to show the number of atoms of each . CH 2 (methylene) has one carbon atom and two hydrogen atoms. Computed by PubChem 2.Chemical formulas tell you how many atoms of each element are in a compound, and empirical formulas tell you the simplest or most reduced ratio of elements in a compound. The molecular mass of 180 u must be some multiple of this number.This same approach may be taken considering a pair of molecules, a dozen molecules, or a mole of molecules, etc.For example, the molecular formula C 4 H 10 tells us there are 4 carbon atoms and 10 hydrogen atoms in a molecule, but it doesn’t distinguish between butane and isobutane.
Calculadora de formula empirica y molecular de un compuesto
The non-whole number empirical formula of the compound is Fe1O1.This program determines the molecular mass of a substance. The empirical formula mass of CH₂O is 30.Although these distinct compounds all have the same molecular formula, only one (A) can be called hexane.There are 4 easy steps to find the molar mass of CH2 based on its chemical formula.Ammonia is a compound of nitrogen and hydrogen as shown below: Figure 6. If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. And for each compound, they all have a molecular formula, but some can be similar, and those are called isomers, which are common in organic chemistry.Boiling Point : 39. Este hidrocarburo tiene cuatro átomos de hidrógeno unidos a un par de átomos de carbono que están conectados por un doble enlace.67 g O / 16 (g/mol) O = 0. The formula of the five-carbon alkane pentane is \(\ce{C5H12}\) so the difference in hydrogen content is 4.0 mole of H 2 O is composed of 2.If the empirical formula is CH₂O, the actual formula is (CH₂O)n or CnH 2nOn, where n = 1, 2, 3, . In the CH 2 Lewis structure, there are two single bonds around the carbon atom, with two hydrogen atoms attached to it, and on the carbon atom, there is . Molecular Weight . For example, (\(\ce{CH4}\)) is a molecule formula of methane which means there is one carbon and four hydrogen atoms in a methane .
Empirical, molecular, and structural formulas
VSEPR theory also predicts, accurately, that a water molecule is ‘bent’ at an angle of approximately 104. Fórmula molecular = 2 · fórmula mínima. Modify: 2024-04-19. A compound is determined to have a molar mass of 58. A molecular formula shows only the kinds and numbers of atoms in a molecule.0 moles of hydrogen and 1. If the molecular (or molar) .Condensed Structure Formula. How then are we to name the others? 1: The molecular formula for ammonia. Its condensed structural formula is CHCl3 CHCl 3.2 (PubChem release 2021.L'éthylène (ou éthène) est un hydrocarbure à deux atomes de carbone, de formule C 2 H 4, ou plus précisément CH 2 =CH 2 (avec une double . The latter amount is most convenient and would simply involve the use of molar masses instead of atomic and formula masses, as demonstrated Example 3.We use several kinds of formulas to describe organic compounds.Condensed Structural Formula; methane: methyl: CH 3 – ethane: ethyl: CH 3 CH 2 – propane: propyl: CH 3 CH 2 CH 2 – isopropyl (CH 3) 2 CH– butane: butyl* CH 3 CH 2 . Enter a chemical formula to calculate its molar mass and elemental composition: Molar mass of CH2 (Methylene) is 14.

Molecular formulas give the kind and number of atoms of each element present in the molecular compound. Added Jun 9, 2014 by WebTester in Chemistry. Enter the molecular formula of the substance. An empirical formula instead shows the ratio between atoms of different elements. Fórmula molecular del benzoato de metilo = C8H8O2. 2015Empirical and Molecular Formulas - Chemistry | Socratic Determining Formula - Chemistry | Socratic Afficher plus de résultats Create: 2004-09-16. The empirical formula mass for . Convert between CH2 weight and moles.
Structure de Lewis CH2 en 5 étapes (avec images)
Ethylene - Density and Specific Weight vs.
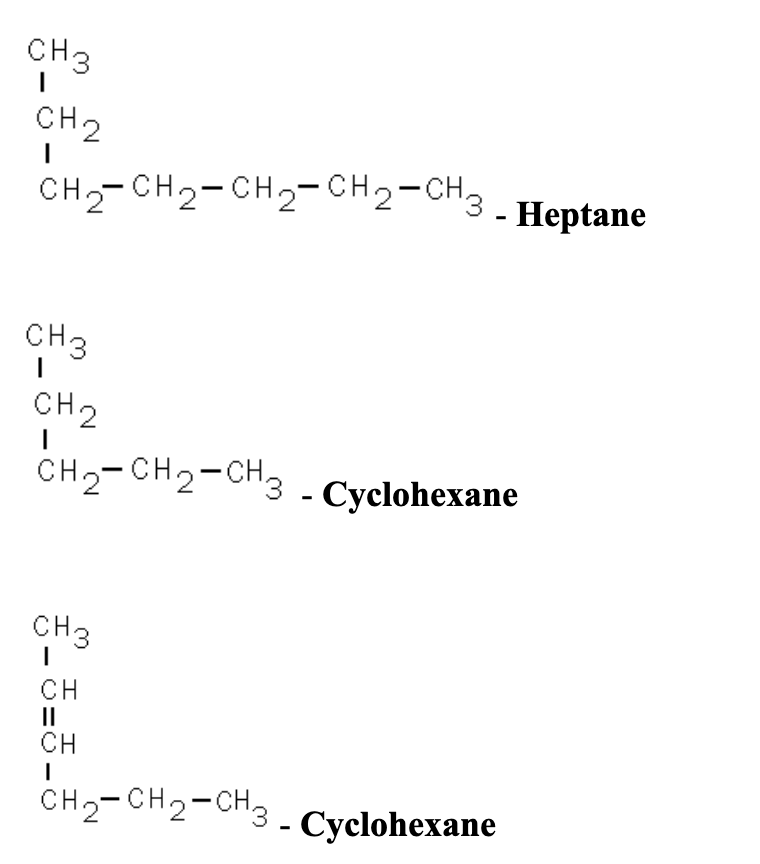
The molecular formula is then obtained by multiplying each subscript in the empirical formula by n, as shown by the generic empirical formula A x B y: \[\left(A_x B_y\right)_n=A_{n x} B_{n y} \nonumber \] For example, consider a covalent compound whose empirical formula is determined to be CH 2 O. In condensed structure formulas, the C-H bonds are omitted and all the H atoms attached to a certain carbon (or other atoms) are usually shown as a group like CH 3, CH 2, NH 2, OH. Thus, H 2 O is composed of two atoms of hydrogen and 1 atom of oxygen. C 5 H 12; CH 3 (CH 2) 3 CH 3; Synonyms.we were quoted an empirical formula of CH_2. Each carbon dioxide molecule contains one carbon atom and two oxygen atoms, bound to each other by .The molecular formula is always a whole number multiple of the empirical formula.The structures in Table 2.Cycloalkanes only contain carbon-hydrogen bonds and carbon-carbon single bonds, but in cycloalkanes, the carbon atoms are joined in a ring. A flow chart is shown that is composed of six boxes, two of which are connected together by a right facing arrow and located above two more that are also connected by a right-facing arrow.
Manquant :
molecular formulaEmpirical formula and molecular formula
1665 and round to whole values, then we get the following indices for . The first step to finding the molar mass of Methylene .Total des électrons de valence dans la molécule CH2 = électrons de valence donnés par 1 atome de carbone + électrons de valence donnés par 2 .CH 3 (CH 2) 8 CH 3. They are very different compounds, yet both have the same empirical formula of CH2O CH 2 O. For example, the molecular formula of glucose is C6H 12O6, and we do not simplify it into CH 2O.Lewis Structure Finder.Click here:point_up_2:to get an answer to your question :writing_hand:the empirical formula of a compound is ch2 its molecular mass is 70 gcdot mol If we now divide the found numbers of moles by the minimum value of 0.

La molécula no puede rotar alrededor del doble enlace y todos los átomos están en el mismo plano.
Pentane
Temps de Lecture Estimé: 2 min N-pentane appears as a clear colorless liquid with a petroleum-like .Determining Empirical Formulas.As the name suggests, an empirical formula mass is the sum of the average atomic masses of all the atoms represented in an empirical formula.As long as the molecular or empirical formula of the compound in question is . PENTANE ; n-Pentane ; 109-66-0 ; Pentan ; Skellysolve A ; View More. Percentages can be entered as decimals or percentages (i.and so, since {empirical formula}xxn=underbrace(56*amu)_quoted molecular mass (12. The two nonbonding electron pairs on oxygen are located in the two remaining sp3 orbitals.and molecular formula is C_4H_8. To calculate the empirical formula, enter the composition (e.
Chemical or Molecular Formula for Carbon Dioxide
Estructura y propiedades Descripción orbital del enlace entre el etileno y un metal de transición. Methylene radical.

50% can be entered as . There are three main types of chemical formulas: empirical, molecular and structural.Finalmente, debe establecerse la fórmula molecular del benzoato de metilo. For example, the molecular formula C 4 H 10 tells us there are 4 carbon atoms and 10 hydrogen atoms in a molecule, but it doesn’t distinguish between butane and isobutane.What is the empirical formula of propene? Of polypropene?12 mars 2017A compound with the empirical formula CH2 has a molar mass . Methylene is fluid-like chloroform in appearance and odour, but differing in its boiling . Since the moles of O O is still not a whole number, both moles can be multiplied by 2, while rounding to a whole number.comEthylene | Structure, Sources, Production, Uses, & Factsbritannica.