Ch3cl molecule type
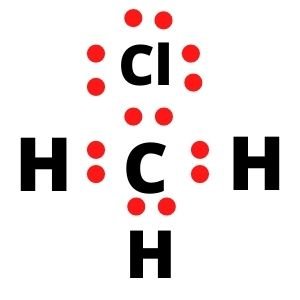
Ceci traduit l'effet répulsif prépondérant des paires libres par rapport aux paires liantes. A step-by-step explanation of how to draw the CH3Cl Lewis Dot Structure (Chloromethane).2 ): (CC BY-NC-SA; anonymous) The two oxygens are double bonded to the sulfur. Ion-ion interactions. Il existe une .CH3Cl lewis structure has a Carbon atom (C) at the center which is surrounded by three Hydrogen atoms (H) and one Chlorine atom (Cl). Dispersion forces.
Structure de Lewis CH3Cl en 6 étapes (avec images)
Crystalline substances can be described by the types of particles in them and the types of chemical bonding that take place between the particles.

Cl is one of the more electronegative elements. Il existe néanmoins une infinité de combinaisons possibles (les plus petites .The CH3Cl is a Penta atomic molecule with a bond angle of 109. Explanation: CH3Cl belongs to the C3v point group.CH3Cl, ou chlorure de méthyle, est une molécule polaire.Classes of Crystalline Solids. IUPAC Standard InChIKey: NEHMKBQYUWJMIP-UHFFFAOYSA-N. Geometry of Molecules.
Chlorométhane — Wikipédia
It consists of three weakly polar C-H bonds with an electronegativity difference of 0. IUPAC Standard InChIKey: NEHMKBQYUWJMIP-UHFFFAOYSA-N Copy; CAS Registry Number: 74-87-3; Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript.Formula: CH 3 Cl. According to Valence shell .Maintenant, dans la molécule CH3Cl, vous devez mettre les paires d’électrons entre les atomes de carbone (C) et de chlore (Cl) et entre les atomes de carbone (C) et d’hydrogène (H).The formal charge is a way of computing the charge distribution within a Lewis structure; the sum of the formal charges on the atoms within a molecule or an ion must equal the . What types of I.Expert-verified. Each water molecule accepts two hydrogen bonds from two other water molecules and donates two hydrogen atoms to form hydrogen bonds with two more water molecules, producing an open, cage like structure. Note: If there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force. ChemSpider ID 6087. Methane, chloro- Monochloromethane. Covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. CH3Cl Lewis Structure, Molecular Geometry, Bond angle and Hybridization. Chloromethane (CH 3 Cl) is a polar molecule. Molecular Weight.CH3Cl has the symmetry elements E, C3, and σv. Send feedback | Visit Wolfram|Alpha. The molecule has a C3 axis that includes the C-Cl bond.

Chloromethane
So, the hybridization number for CHCl3 we got 4 which means it has Sp³ hybridization.

For the CH3Cl structure use the periodic .
Chloromethane
VSEPR structures like this one are often drawn using the wedge and dash notation, in which solid lines represent bonds in the plane of the page, solid wedges represent bonds coming up out of the plane, and dashed lines .
Chloromethane
The oxygens have 2 lone pairs while sulfur had one lone pair.6: Types of Intermolecular Forces- Dispersion, Dipole–Dipole, Hydrogen Bonding, and Ion-Dipole .41K subscribers.
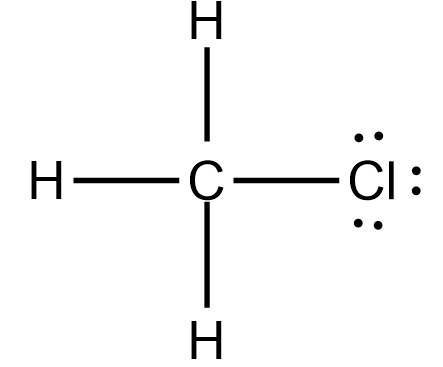
Il existe 3 liaisons simples entre l’atome de carbone (C) . METHYL CHLORIDE.
Chloromethane (CH3Cl) Lewis Structure
IUPAC Standard InChI: InChI=1S/CH3Cl/c1-2/h1H3.

Each fluorine has three lone pairs.
CH3Cl Lewis Structure (Chloromethane)
17 The molecular structure of the methane molecule, CH 4, is shown with a tetrahedral arrangement of the hydrogen atoms.La structure CH3-Lewis a un atome de carbone (C) au centre qui est entouré de trois atomes d’hydrogène (H).Molecular Formula. (Valence electrons are the number of electrons present in the outermost shell of an atom).For the CH3Cl structure use the periodic table to find the total numb.10: Electronegativity and Bond Polarity is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. More details: Names. Domaine spectral: UV . dipole-dipole forces intermolecular forces II. There are 3 Hydrogen atom (s), 1 Carbon atom (s), and 1 Chlorine atom (s). The more ionic, the higher the lattice energy. Is CHCl3 tetrahedral? The shape of CHCl3 molecule is tetrahedral due to the presence of sp3 hybridisation. Les formes des molécules dépendent des facteurs suivants : 1) Le nombre d’atomes dans la molécule.
11: Intermolecular Forces and Liquids
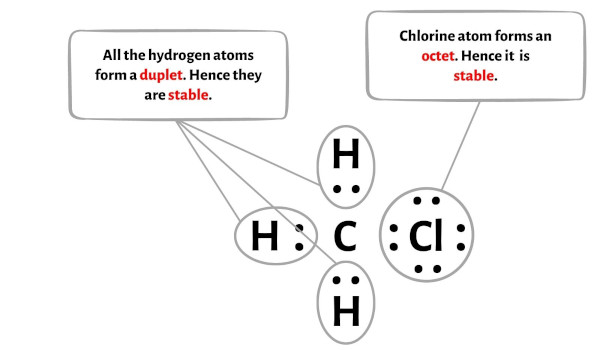
Step #1: Calculate the total number of valence electrons. Les atomes ne peuvent pas se lier entre eux n’importe comment.5° which gives the molecule a bent shape.CH 3 Cl has 4 + 3 + 7 = 14 valence electrons. L’une des trois forces de van der Waals est présente dans toutes les phases condensées, quelle que soit la nature des atomes ou des molécules qui composent la substance. Étape 1: vérifier que les atomes liés sont différents (une liaison entre deux atomes identiques est nécessairement . 102K views 10 years ago. Étape 4 : Rendre les atomes externes stables
List out the symmetry elements of ch3cl?
The trick for calculating the type of hybridization. Applications include the study of biomolecule:ligand complexes, free energy calculations, structure-based drug design and refinement of x-ray crystal . Properties and several examples of each type are listed in the . Sulfur has one lone pair.Déterminer si une liaison est polarisée à partir de l’électronégativité des atomes.Les molécules.Les transitions électroniques dans la molécule sont à l'origine de l'absorption du faisceau incident lors de l'analyse dans le domaine UV-visible effectué à l'aide du spectrophotomètre. It is polar because it .January 1, 2021. Monoisotopic mass 49. There are two bonding pairs and one lone pair, so the structure is designated as AX 2 E. In VSEPR theory, we calculated the total electron pairs in the last step.
hydrogen bonding are exerted by CH3C1 III.
Le CH3CL est-il une molécule polaire
For CHCl3, it came out to be 4.Hello Guys!Today in this video, we are going to learn the hybridization of the CH3CL molecule. Molécule CH 4 NH .Lewis Structure Finder. The formation of covalent bonds is . Molecular weight: 50. The molecules in liquid C 12 H 26 are held together by _____. The bond angle in the CHCl3 molecule is around 109. Transition σ→σ*. There is a single . These four bonds take eight electrons, and the . Hydrogen bonding. Chloromethane or Methyl chloride having a molecular . Molecular Formula CHCl. Covalent bonds can be broken if energy is added to a molecule.Chloromethane (CH 3 Cl) contains one carbon atom, three hydrogen atoms and one chlorine atom. From the table below, we can predict hybridization using total electron pairs or steric numbers.IUPAC Standard InChI: InChI=1S/CH3Cl/c1-2/h1H3 Copy. There are four types of crystals: (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. Forces de dispersion . Computed by PubChem 2.1K views 1 year ago Lewis Structure. Other names: Methane, chloro-; . (A) I only (B) II only (C) I and III only (D) II and III only. 11: Intermolecular Forces and Liquids is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts.The Automated Topology Builder (ATB) and Repository is intended to facilitate the development of molecular force fields for Molecular Dynamics or Monte Carlo simulations of biomolecular systems.Yes, Methyl chloride (CH3Cl) or Chloromethane is a polar molecule. Le chlorure de méthyle a un atome de carbone central entouré de trois atomes d'hydrogène et d'un atome de chlore. Four fluorenes are bonded to a central sulfur.CH3Cl is a fairly polar molecule. Les atomes se lient entres eux et forment des assemblages qu’on appelle des molécules. The C-Cl covalent bond shows unequal electronegativity because Cl is more electronegative than carbon causing a separation in charges that results in a net dipole.In a polar covalent bond, sometimes simply called a polar bond, the distribution of shared electrons within the molecule is no longer symmetrical (see figure below). It is a chemical formula for Chloromethane.

5 atom(s) - 3 Hydrogen atom(s), 1 Carbon atom(s), and 1 Chlorine atom(s) How many chemical bonds and what .CH 3 Cl is the chemical formula for chloromethane, also known as methyl chloride.
Chloromethane Structure
So, H = 4 + 0 = 4.35 units and one strongly polar C-Cl bond due to an electronegativity . 4: In the polar covalent bond of HF HF, the electron density is unevenly distributed. To understand the .Temps de Lecture Estimé: 6 min
Chloromethane
Ion-dipole interactions.A step-by-step explanation of how to draw the CH3Cl Lewis Dot Structure (Chloromethane). An explanation of the molecular geometry for the CH3Cl (Chloromethane or Methyl chloride) including a description of the CH3Cl bond . Average mass 50. Cette force attractive est appelée la force de dispersion de . The Chloromethane molecule contains a total of 5 atom (s). Liaisons chimiques 56 - - - - Les composés qui possèdent des mêmes constituants mais des structures La forme des molécules En raison de l’arrangement des atomes dans les molécules covalentes qui ont des positions et des directions exactes, ce qui fait certains composés ont chacun différentes formes. Carbon goes in the middle and is bonded to three hydrogen and one chlorine atom.In general, intermolecular forces can be divided into several categories. Cela indique que ces atomes sont chimiquement liés les uns aux autres dans une molécule CH3Cl. It is an odorless, colorless, flammable chemical compound that exists as a gas .n/a Ensembl ENSG00000278567 ENSG00000277632 ENSG00000274221 n/a UniProt P10147 n/a RefSeq (mRNA) NM_002983 n/a RefSeq (protein) NP_002974 n/a Location . This designation has a total of three electron pairs, two X and one E. The three-fold axis may be more evident if you look down the C-Cl bond . Examine the following list and see if you can explain the observed values by way of ionic attraction: LiF .
Liaison polarisée
In order to draw the lewis structure of CH3Cl, first of all you have to find the total number of valence electrons present in the CH3Cl molecule. It has the symmetry element E, a C3 axis, and three σv planes. The structure of CH3Cl is.Chemical Formula Description.Trichloroethane ( C2H3Cl3) may refer to either of two isomeric chemical compounds: 1,1,1-Trichloroethane (methyl chloroform, CCl 3 CH 3) 1,1,2-Trichloroethane (vinyl trichloride, .10K views 2 years ago. Question: What kind of intermolecular forces act between a chloromethane CH3Cl molecule and a chloride anion?\. There is a higher density (red) near the fluorine atom, and a lower density . The C-Cl bond has a large difference in electronegativity compared to the H-C bonds. Dipole-dipole interactions.