Charge of cl2

What is the number of cl2 oxidation here? The oxidation number of chlorine .
orgRecommandé pour vous en fonction de ce qui est populaire • Avis
Cl2 Lewis Structure, Geometry, Hybridization, and Polarity
Depending on where you get your data from, the theoretical value for lattice enthalpy for AgCl is anywhere from about 50 to 150 kJ mol -1 less than the value that comes from a Born-Haber cycle.Chlorine | Cl2 | CID 24526 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, .Chlorine gas (Cl2) is a green yellow gas with a pungent odour and a molecular weight of 70. IUPAC Standard InChI: InChI=1S/Cl2/c1-2.Redox reactions are identified per definition if one or more elements undergo a change in oxidation number. A polar covalent bond is a covalent bond in which the . For example: ZnCl4 {2-} or NH2NH3 {+}. Using a simple, general trend for the ionic charge for elements on the Periodic Table, in this video we find the ionic charge for Chlorine . Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure .
Balancing Redox Reactions
On the right, the sodium ion only has 10 electrons and a 1+ charge.Gas extraction.Cl2 is free of charge.Enter the formula of a chemical compound to find the oxidation number of each element. Write down the unbalanced equation ('skeleton equation') of the chemical reaction. How many ions are in CL? two There are two chloride ions in the formula. Make electron gain equivalent to electron lost.Therefore, the overall charge of the right side is +2. Chlorine, on the other hand, has -1 (generally not always) charge when it is present in its ionic form. IUPAC Standard InChIKey:KZBUYRJDOAKODT-UHFFFAOYSA-N Copy.Step 1: Find the total valence electrons in one molecule of chlorine dioxide. It is 20 as chlorine has 7 valence electrons and oxygen has 6 valence electrons.1 is called a polar covalent bond.Regarder la vidéo13:23🚀To book a personalized 1-on-1 tutoring session:👉Janine The Tutorhttps://janinethetutor.The oxidation state of a simple ion like hydride is equal to the charge on the ion—in this case, -1. You can see the number of bonding electrons and nonbonding electrons for each atom of COCl2 molecule in the image given below. Molecular weight: 70. Calculate the formal charge of chlorine in the molecules Cl2, BeCl2, and . Valence electrons = 7, Lone pair .

Federal government websites often end in . We are assuming (and shall do all through this page) that all the ions recorded have a charge of 1+. This is a redox reaction.
Structure de Lewis Cl2 en 6 étapes (avec images)
Mark charges on atoms if there are charges. Total number of . 47K views 5 years ago. O: Cl 2 + 2H 2 O → 2HOCl + 2H + + 2e- R: Cl 2 + 2H + + 2e-→ 2HCl .In this paper, we present an alternative picture for the electronic structure of dihalogen molecules and for the physical origin of the “charge-shift bonding” effect. A chlorine atom has the following electron configuration: Cl: 1s 2 2s 2 2p 6 3s 2 3p 5.1, the formal charge on the nitrogen atom is . calculate the formal charges of the atoms CO, CO_2, and CO_3^2-Determine the formal charge on each atom in the nitrite ion. A step-by-step explanation of how to draw the Cl2 . The sodium loses an electron, and the chlorine gains that electron. An official website of the United States government.Chlorine dioxide is a chemical compound with the formula ClO 2 that exists as yellowish-green gas above 11 °C, a reddish-brown liquid between 11 °C and −59 °C, and as bright .
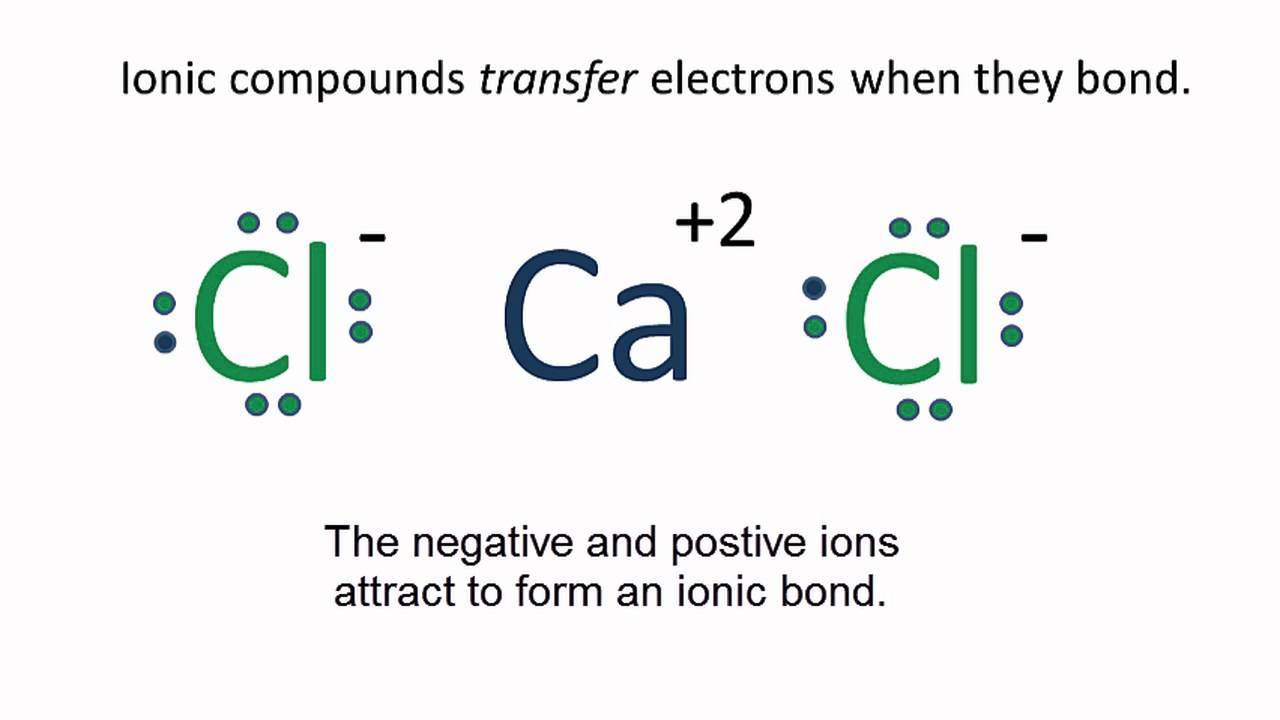
The upper states at 67700 and 75000 cm-1 are considered to be 1 g states and, therefore, are not observed in absorption from the ground state. It is also used to make hundreds of consumer products from paper to paints, and from textiles to insecticides. If sodium metal and chlorine gas mix under the right conditions, they will form salt. 2013Temps de Lecture Estimé: 1 minUpdated on July 18, 2022.Date de publication : 23 nov. Alternatively, the sum of the oxidation states in a neutral compound is zero. This implies that each chlorine atom has a net -1 charge. All reactants and products must be known. IUPAC Standard InChIKey: . The first shows common element charges, while the second shows all the element charges for the first 45 elements (most common charges in .7 Electrolysis. Enter just an element symbol to show the common and uncommon oxidation states of the element. This is not a redox reaction, since oxidation numbers remain unchanged for all elements.Cl2 Lewis Structure
Chlorine
(In table salt, this electron comes from the sodium atom. Gallium is oxidized, its oxidation number increasing from 0 in Ga ( l) to +3 in GaBr 3 ( s ).Auteur : Wayne Breslyn Typical is the conversion starting from the ore . Using Equation 4.In order to calculate the formal charges for ClO2- we'll use the equation:Formal charge = [# of valence electrons] - [nonbonding val electrons] - [bonding el.Overview
Chlorine
Chart of Common Charges of Chemical Elements
Only one more electron is needed to achieve an octet in chlorine’s valence shell.Thus, we calculate formal charge as follows: formal charge = # valence shell electrons (free atom)−# lone pair electrons− 1 2# bonding electrons formal charge = # valence shell electrons (free atom) − # lone pair electrons − 1 2 # bonding electrons. But charge on chlorine varies from -1 to +7. Here is how you know. You can use this chart to predict whether or not an atom can bond .
Manquant :
chargePeriodic Table with Charges - 118 Elements - Science .Notes: Isotopes are atoms of the same element (and so with the same number of protons), but with different masses due to having different numbers of neutrons. To find the correct oxidation state of Cl in Cl2 (Chlorine gas), and each element in the .
Step 5: Multiply both sides of both reactions by the least common multiple that will allow the half-reactions to have the same number of electrons and .For calculating the formal charge, you have to use the following formula; Formal charge = Valence electrons – (Bonding electrons)/2 – Nonbonding electrons.orgPrintable Periodic Table - Element Charges - Science .
Balancing redox reactions by the ion-electron method
Unités du SI et CNTP, sauf indication contraire.) The electron configuration of the new species that results is as . Copy Sheet of paper on top of another sheet. The reducing agent is Ga ( l ).1: They have been interpreted Asundi and Venkateswarlu, 1947 as being due to transitions from stable excited states at 58000 (possibly F), 67700 and 75000 cm-1 to the repulsive states arising from 2 P + 2 P. But when it is present in its ionic form then chlorine have -1(generally not always) charge on it. Rule 4: Specify the identity of the metal.Auteur : OneClass
Oxidation States (Oxidation Numbers)
> Chlorine in HClO_4 (hydrogen is bonded to O) Calculate the formal charge of the chlorine in chlorate.
Dichlore — Wikipédia
What is the charge of CL2? Cl2 does not have any charge.2K views 3 years ago Chapter 4 | SOLUTION MANUAL for Chemistry: Atoms First | OpenStax™️. Enter the formula of a chemical compound to find the oxidation number of each element.com🚀More proven OneClass Services you might be interested in:👉One. This reaction is highly favorable because of the electrostatic attraction between the particles. It is used to treat drinking water and swimming pool water. Skip to Content Go to accessibility page Keyboard shortcuts menu. Contents Contents. Il est 2,5 fois plus dense que l'air.

Process chemistry. We must add 5 electrons to the left side of the equation to make sure that both sides of the equation have equal charges of +2. Rule 3: Specify the identity, number, and as appropriate, isomerism of the ligands present in alphabetical order by ligand name.

Manquant :
charge It doesn't matter what the charge is as long as it is the same on both sides.It is the result of Na+ ions and Cl- ions bonding together (Figure 4.The formal charge of chlorine in the Cl 2 can be calculated by-Formal charge = Valence Electrons – Unbonded Electrons – ½ Bonded Electrons. We can double-check formal charge calculations by determining the sum of the formal .In the oxidation number change method the underlying principle is that the gain in the oxidation number (number of electrons) in one reactant must be equal to the loss in the oxidation number of the other reactant.What charge is cl2?
) Molecule Lewis Structure.gov means it’s official. A net ionic charge can be specified at the end of the compound between { and }.Regarder la vidéo1:48Cl2 Lewis Structure - How to Draw the Dot Structure for Cl2 - YouTube.Metallic sodium, Na, and chlorine gas, Cl2, are used in numerous applications, and their industrial production relies on the large-scale electrolysis of. In other words, treating the AgCl as 100% ionic underestimates its lattice enthalpy by quite a lot. The electrons lost in the oxidation .
THE MASS SPECTRA OF ELEMENTS
The shared pair of electrons belong to both Chlorine atoms, meaning that each Chlorine atom has 18 electrons and 17 protons which is 1 more electron than the total number of protons. In this process, the feedstock is treated at 1000 °C with carbon and chlorine gas, giving titanium tetrachloride. Check the stability and minimize charges on atoms by converting lone pairs to bonds to obtain best lewis structure.Here are two charts. Oxygen in peroxides: . is a twoChlorine atom molecule with one negative charge, made up of twoChlorine atoms. Because Group 1 metals always have an oxidation state of +1 in their compounds, it follows that the hydrogen must have an oxidation state of -1 (+1 -1 = 0).The Lewis electron structure for the NH 4+ ion is as follows: The nitrogen atom shares four bonding pairs of electrons, and a neutral nitrogen atom has five valence electrons. Chlorine is a diatomic molecule and contains only two chlorine atoms. Table of contents.
Lattice Enthalpies and Born Haber Cycles
It is a chemical element that belongs to the halogen group and has disinfectant, .
Manquant :
chargeElement Charges Chart
Rule 2: When multiple isomers are possible, designate the particular isomer in italics at the front of the name of each complex. Le dichlore ( Cl2) est un gaz jaune-vert ( chloros signifie « vert » en grec) dans les conditions normales de température et de pression.1: They have been interpreted Asundi and Venkateswarlu, 1947 as being due to transitions from stable excited states at 58000 (possibly F), 67700 and 75000 cm-1 to the repulsive . A net ionic charge can be specified at the end of the compound between { and .










