Chemical equilibrium nacl
The common-ion effect is used to describe the effect on an equilibrium involving a substance that adds an ion that .The solubility product ( Ksp) is used to calculate equilibrium concentrations of the ions in solution, whereas the ion product ( Q) describes concentrations that are not necessarily at equilibrium. If additional chloride ions (the “common ion”) are added, then Le . Each set of panels shows the changing composition of one of the three reaction mixtures as a function .
Dissociation of NaCl
These solutions conduct electricity due to the mobility of the positive and negative ions, which are called cations and anions respectively. This law states that the reaction quotient, Q, will be equal to a constant when a system reaches chemical .2, for example, vary by 60 orders of magnitude.
Na {+} + Cl {-} = NaCl
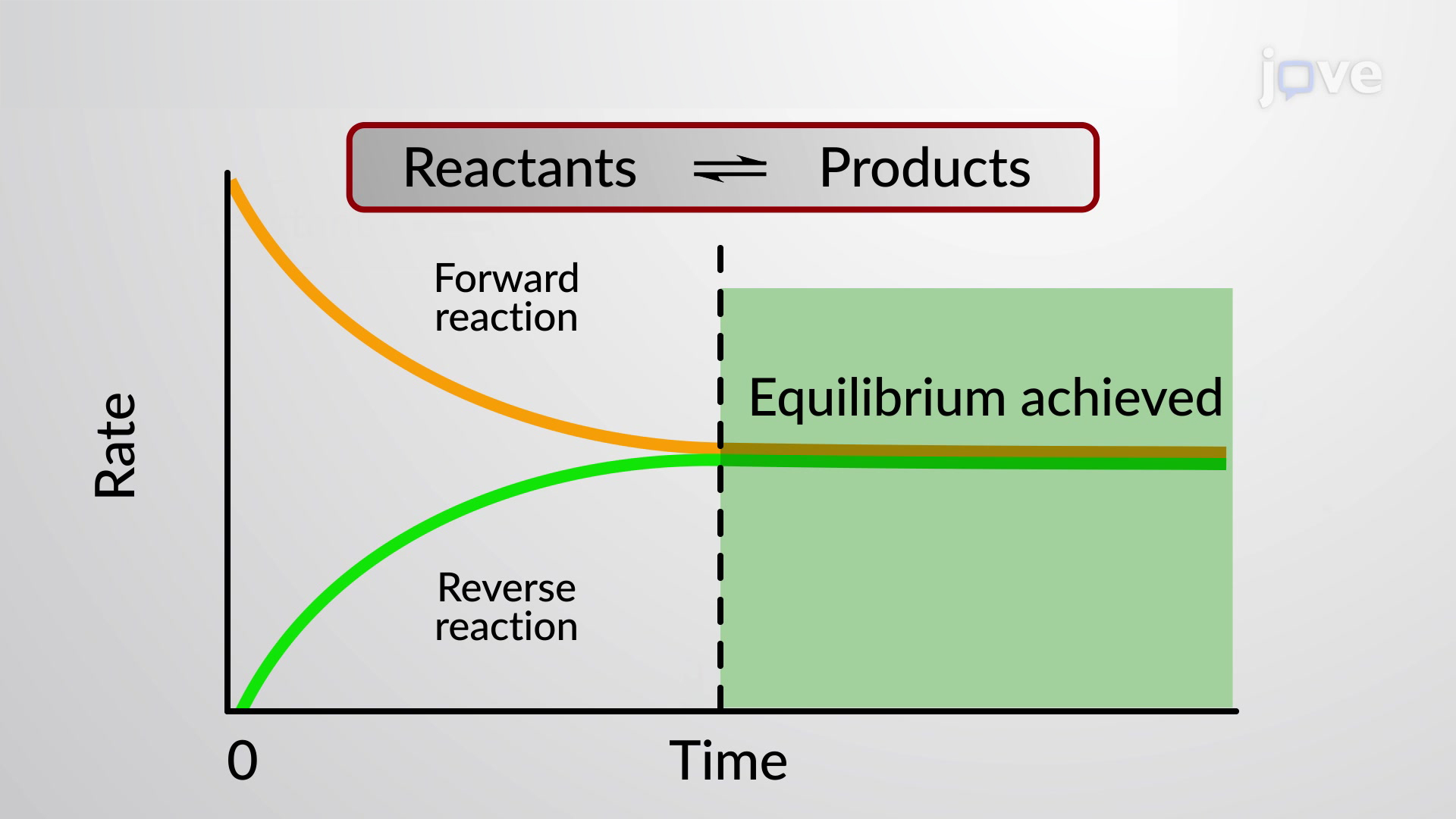
5 is true for any pair of opposing reactions regardless of the mechanism of the reaction or the number of steps in the mechanism.
HCl + NaOH = NaCl + H2O
These salts will not be considered in this concept.10 M, the initial amount that was put into solution. NaCl + AgNO3 = NaNO3 + AgCl. Calculate and compare Q and K values.
Na + CL = NaCL
When an ionic solid such as silver chloride (\(AgCl\)) dissolves in water, it breaks up into ions that move apart from each other and become solvated by water molecules, and the following .NaCl(s) ⇌ Na+(aq) + Cl–(aq) In a saturated solution, the ions in solution are in equilibrium with the solid phase.comRecommandé pour vous en fonction de ce qui est populaire • Avis
Chemical equilibrium
NaCl = Na + Cl is a Decomposition reaction where one mole of aqueous Sodium Chloride [NaCl] decomposes into one mole .HCl + NaOH = NaCl + H2O is a Double Displacement (Acid-Base) reaction where one mole of aqueous Hydrogen Chloride [HCl] and one mole of aqueous Sodium Hydroxide .
CaCO3 + NaCl = Na2CO3 + CaCl2
Pour approximately 5 mL of the saturated NaCl solution over the filter paper in the funnel, which should be placed over a test tube.
12: Equilibrium and Le Chatelier's Principle (Experiment)
Chemical equilibrium can be attained whether the reaction begins with all reactants and no products, all products .Calcium goes from 0 to +2, losing 2 electrons (reduction). [Acetic acid] + [acetate] = 0.For obvious reasons, we call the double arrow, \(\rightleftharpoons\), an equilibrium arrow.comHow to Balance: Na + Cl2 = NaCl - YouTubeyoutube.A judicially selected equilibrium constant of NH 4 Cl, which is consistent with .Thermodynamic Properties of the NaCI + H20 System II. If such a stress is applied, the reversible reaction will .10 M, but the total of the two species must equal 0.TITLE EXPERIMENT 3 : CHEMICAL EQUILIBRIUM: LE CHATELIER`S PRINCIPLE PROGRAM & GROUP: AS113 & RAS1133A COURSE: CHM 213 DATE EXPERIMENT: 2/10/2018 NAME MATRIX NO MUHAMMAD IRFAN BIN AZWARI 2017256762 MUHAMMAD IQBAL BIN INDRA MUFELI 2017248854 JESHUA JEROD JUNIK . To learn more about NO 2 (g), you decide to study this pollutant spectroscopically (by light absorption). Chemistry in Context December 2, 2019.
NaCl = Na + Cl
Although a system at equilibrium appears .comLab Report on Le Chatelier's Principle - 1372 Words | .
Na2CO3 + HCl = NaCl + H2O + CO2
Quasi-equilibrium chemical evolution in starless cores
This page titled 7. The equilibrium constant for a dissolution reaction, called the solubility product ( Ksp ), is a measure of the solubility of a compound.NaCl: Strong acid \(+\) Weak base: Acidic: NH 4 Cl: Weak acid \(+\) Strong base: Basic: NaF: Salts formed from the reaction of a weak acid and a weak base are more difficult to analyze because competing hydrolysis reactions between the cation and the anion.Le Chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed.Thus, the equilibrium constants for reactions 6.
At the same rate that solid NaCl produces aqueous NaCl (dissolved salt), the dissolved salt is recrystallizing to form more solid NaCl.0 K and then open the stopcock on the cell to equilibrate the . To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button.Le Chatelier's Principle states that if a stress is applied to a reversible reaction at equilibrium, the reaction will undergo a shift in order to re-establish its equilibrium.15 K, using the flow-cloud-point method (Zhang et al. Calculate its Ksp. Salt hydrolysis is defined.
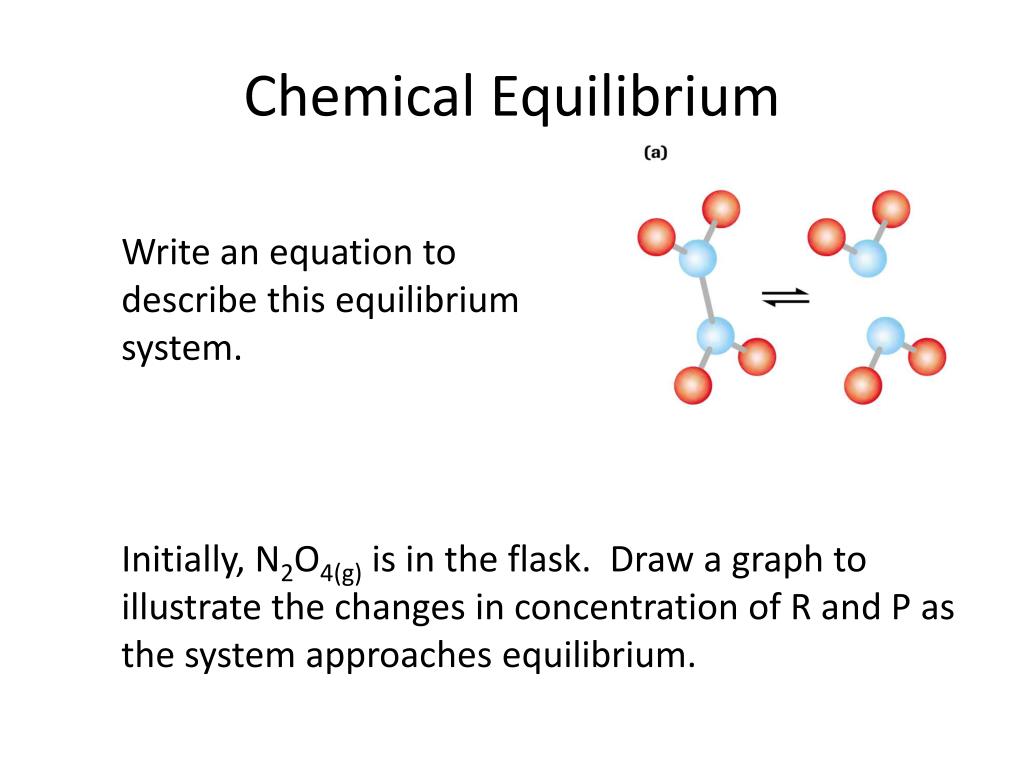
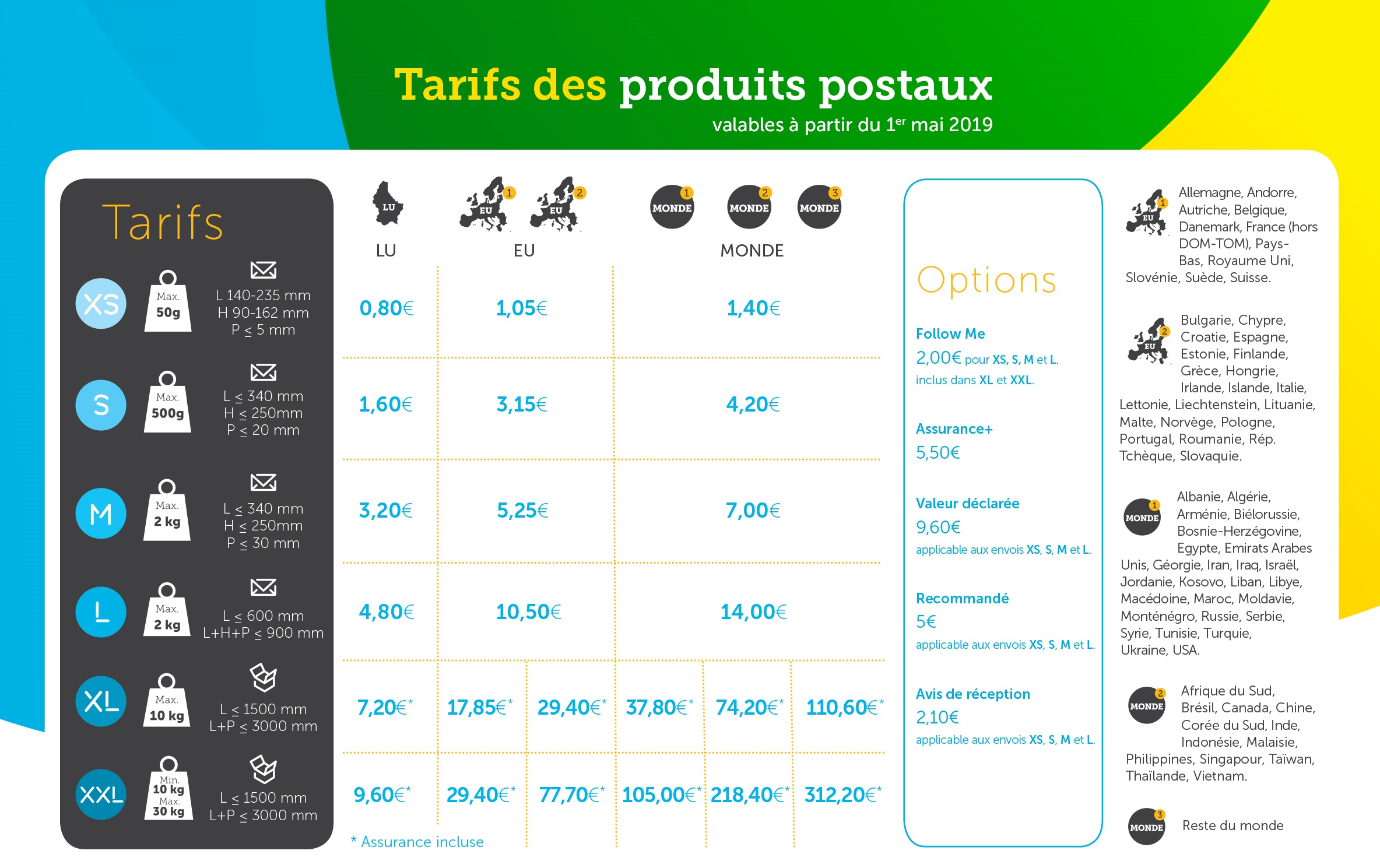
Solid–liquid equilibrium (SLE) values were experimentally determined for the NaCl–NH 4 Cl–H 2 O system in the temperature range from 293. CaCl2 + Na2CO3 = 2 NaCl + CaCO3.36 × 10 −4 g/100 mL. Its solubility in water at 25°C is 7. The three reaction systems (1, 2, and 3) depicted in the accompanying illustration can all be described by the equation: 2A ⇌ B (15. Explain the importance of the equilibrium constant. However, the lower the temperature .comLab Report 10 - Experiment 10: Le Chatelier's Principle.The solubility of a substance in a solvent is defined as the greatest amount that will dissolve in equilibrium in a specified volume of solvent at a particular temperature. The values of K shown in Table 15. For any general reaction, aA + bB ⇌ cD + dD (15.11 is correctly identified as β4, where. Calcium Carbonate + Sodium Chloride = Sodium Carbonate + Calcium Chloride., 1998, Journal of Chemical and Engineering Data, 43, 32–37).Chemists have found that there is a mathematical relationship that exists between the concentration of the reactants and products, once equilibrium has been reached, that is independent of the initial concentration of the participants. Collect the filtrate in the test tube, and remove the funnel. Archer Chemical IUnl!ric!I .Sodium Bromide + Chlorine = Bromine + Sodium Chloride. Predict relative amounts of reactants and products based on equilibrium . A reversible reaction is a reaction in which both the conversion of reactants to products (forward reaction) and the re-conversion of products to reactants (backward reaction) .
Step 4: Substitute Coefficients and Verify Result. Na2CO3 + 2 HCl = 2 NaCl + H2O + CO2.Sodium chloride / ˌsoʊdiəm ˈklɔːraɪd /, [8] commonly known as edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and .chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and products occurs.I get the equation: NaOH +HCl HX2O +NaCl N a O H + H C l H X 2 O + N a C l. The chemical responsible for the brown air throughout the Los Angeles area is NO 2 (g). Handout - Lab Report - 2/23/18 Experiment: .The relationship shown in Equation 15. Since there is an equal number of each element in the reactants and products of Na + Cl = NaCl, the equation is balanced. Facebook LinkedIn X Pinterest Email This animation shows how sodium chloride dissolves in . Many parts of the world contain .Sodium chloride, also known as salt, common salt, table salt or halite, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. An overall, or cumulative formation constant, which we designate as βi, describes the addition of i ligands to the free metal ion. Calcium oxalate monohydrate [Ca (O 2 CCO 2 )·H 2 O, also written as CaC 2 O 4 ·H 2 O] is a sparingly soluble salt that is the other major component of kidney stones [along with Ca 3 (PO 4) 2 ].Dissociation of NaCl. Simon Fraser University.comLe Chatelier Lab ANSWERS: Fe3+ and FeSCN2+ Equilibriumyoutube.15 are, respectively, K1, K2, K3, and K4. Equilibrium is the state at which the rate of the forward reaction equals the rate of the .Chemical equilibrium systems obey the law of mass action. Observe and record the results. Sodium Chloride = Sodium + Chlorine.Calculate ion concentrations involving chemical equilibrium.Explain chemical equilibrium. Solid–solid phase . Catalysts do not affect the position of an equilibrium; they help reactions achieve equilibrium faster. Consider the following exothermic reversible reaction at equilibrium: (2.The chemistry of H2O, CO and other small molecular species in an isolated pre-stellar core, L1544, has been assessed in the context of a comprehensive . Berthollet’s reasoning that reactions are reversible was an important step in . Carefully add a few drops of concentrated HCl drop by drop to the test tube.
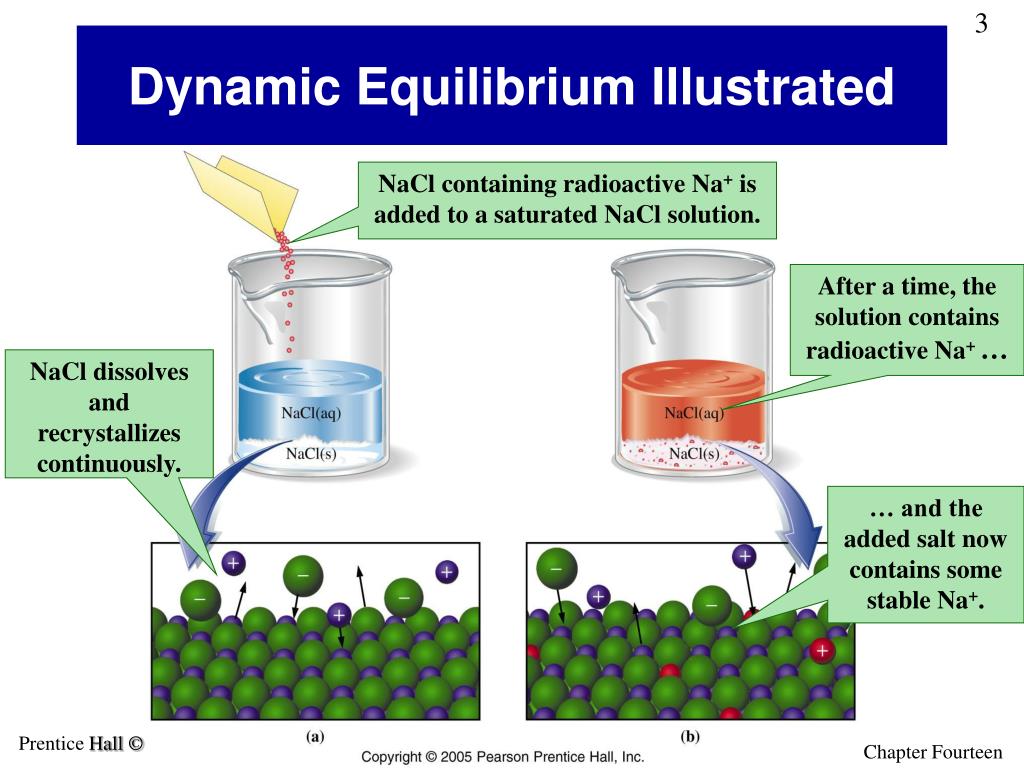
They typically form when NaCl leaches from soils into waters that flow into salt lakes in arid regions that have no natural outlets; subsequent evaporation of these brines force the above equilibrium to the left, forming natural salt deposits.comExperiment 5- Chemical Equilibrium and Le Chatelier’s .
Equilibrium and Le Chatelier's Principle
The direction of shift can be predicted for changes in concentrations, temperature, or pressure.Balance Na+Cl=NaCl | Mathwaymathway.1) aA + bB ⇌ cD + dD.
12: Solubility Equilibria
Shifts in Equilibrium: Le Châtelier’s Principle
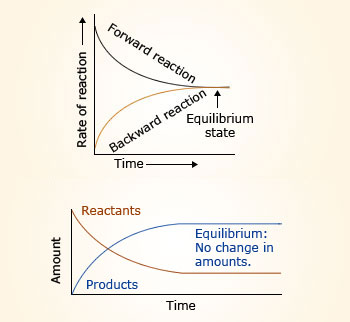
Chemical equilibrium is the state of a system in which the rate of the forward reaction is equal to the rate of the reverse reaction.Substances that give ions when dissolved in water are called electrolytes. CaCO3 + NaCl = Na2CO3 + CaCl2 is a Double Displacement (Metathesis) reaction where one mole of solid Calcium Carbonate [CaCO 3] and two moles of aqueous Sodium Chloride [NaCl] react to form one mole of aqueous Sodium Carbonate [Na 2 CO .Solid–solid phase equilibria in the NaCl–KCl system | The Journal of Chemical Physics | AIP Publishing. Research Article | April 14 2020. The charge balance must account for all positively charged (sodium and hydronium ions) and negatively charged (acetate and hydroxide .A reaction at equilibrium exists in a steady‐state, in which the rate at which a species forms equals the rate at which it is consumed. The Le Chatelier principle tells us that in order to maximize the amount of product in the reaction mixture, it should be carried out at high pressure and low temperature.One way of predicting these concentration shifts is through the use of Le Chatelier's principle: When a stress is applied to a system at equilibrium (a change in .

As the original reactants, on the left side of the equation, react to form products, on the right side of the equation, the products also react to re-form the reactants . To observe the effect of an applied stress on chemical systems at equilibrium. They can be divided into acids, bases, and salts, because they all give ions when dissolved in water.1: Reversible Reactions and Chemical Equilibria is shared under a CC BY-NC-SA 4.Define chemical equilibrium. Recognize chemical equilibrium as a dynamic process. Write the balanced Equation: 3 Ca + 2 P = Ca 3 P 2. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced.1: Equilibrium in reaction: H2(g) +I2(g) ⇌ 2HI(g) H 2 ( g) + I 2 ( g) ⇌ 2 HI ( g). where the blue circles are A A and the purple ovals are B B.
Electrolytes
0 license and was authored, remixed, and/or curated by David Harvey.
Experiment 4 Equilibrium and Le Châtelier’s Principle
The equilibrium constant in Equation 6.