Chemical formula for nitric oxide

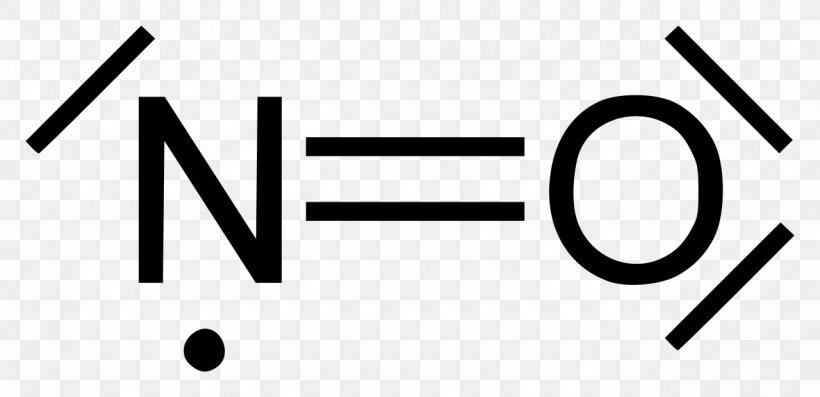
Substance details.CHAPTER 2 The Chemical Properties of Nitric Oxide and Related Nitrogen Oxides Jon M.Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its chemical formula (•N=O or •NO).What's more, nitric oxide as an inhaled prescription has been used in newborns to treat respiratory failure, but NO supplements might be safe while breastfeeding. Concentrations of 60-150 ppm cause immediate irritation of the nose and throat with coughing and burning in the throat and chest. IUPAC Standard InChIKey: MWUXSHHQAYIFBG-UHFFFAOYSA-N. The chemical compound nitric oxide is a gas with formula N O.An important intermediate in industrial chemistry, nitric oxide forms in .Add to MyChemicals Print Friendly Page.Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its chemical formula ( • N=O or • NO). Dinitrogen tetroxide is a colourless solid that is in equilibrium with nitrogen dioxide.This gas is an important signaling molecule in the body of mammals including humans and is an extremely important intermediate in the chemical industry.
Molecular Formula NO; Average mass 30.Nitric oxide (NO) and nitrous oxide (N2O) are not only produced through biological pathways but can also be formed through chemical reactions involving various compounds.All isoforms of NOS utilize l -arginine as the substrate, and molecular oxygen and reduced nicotinamide-adenine-dinucleotide phosphate (NADPH) as co-substrates.Nitrous Oxide: Nitrous oxide is an oxide of nitrogen having the chemical formula N 2 O. Molecular Formula. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its chemical formula . In the normal state NO2 exists in equilibrium with its dimer N2O4. International Journal of Chemical Kinetics 2020 , 52 (5) , 329-340.In atmospheric chemistry, NOx is shorthand for nitric oxide ( NO) and nitrogen dioxide ( NO. It’s a type of acidic oxide. As the tin-nitric acid mixture is heated, , and water are removed leaving ., an unpaired electron ). It is one of the principal oxides of nitrogen.Data at NIST Subscription Sites
Nitric oxide
Understanding the chemical reactions and .Nitric acid is the inorganic compound with the formula H N O 3. nitrogen monoxide. The compound is colorless, but samples tend to acquire a yellow cast .Auteur : The Editors of Encyclopaedia Britannica
Nitric Oxide
Dinitrogen Tetroxide. IUPAC Standard InChIKey: MWUXSHHQAYIFBG-UHFFFAOYSA-N Copy; CAS Registry Number: 10102-43-9; Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed .
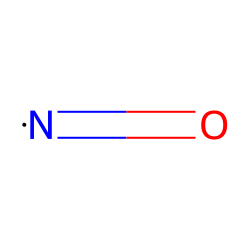
Molecular weight: 30. CASR number: N/A.0061; IUPAC Standard InChI: InChI=1S/NO/c1-2 Copy. Nitric oxide should not be confused with nitrous oxide (N2O), an anesthetic, or with ., it has an unpaired electron, which is sometimes denoted by a dot in its chemical formula, i.
The Nitric Oxide Molecule
5: Nitric oxide: NO-163.In atmospheric chemistry, NO x is shorthand for nitric oxide (NO) and nitrogen dioxide (NO 2), the nitrogen oxides that are most relevant for air pollution. It is easily oxidized to nitrogen dioxide by air. Chemical structure:The nitrous oxide chemical formula is N 2 O.
Nitric oxide
Most nitric acid is used in the manufacture of fertilisers, while some is used in the production of explosives for both military and mining uses. It also has a physiological role as a signaling molecule. It is made, for example, by the reduction of concentrated nitric acid by copper, or reduction of nitrates and nitrites: HNO 3 + 3Cu 3Cu (NO 3) 2 + 4H 2 O + 2NO. Nitrogen Dioxide is a brown gas that is a combined anhydride of nitrous acid and nitric acid.
Nitric oxide synthases: regulation and function
Secondary ChEBI IDs.
![The correct chemical formula of nitrous oxide isa.) \\\\[{{N}_{2}}O\\\\]\\nb ...](https://www.vedantu.com/question-sets/f7a4ec40-786a-4852-aef0-38666c5909602345366317749710186.png)
It is also a toxic air pollutant produced by automobile engines and power plants. It contains nitrogen in its +2 oxidation state. The boiling point of . CAS Registry Number: 10102-43-9. Copy Sheet of paper on top of another sheet. Nitric oxide is a free radical, i. 2 Pb ( N O 3) 2 Lead nitrate → heat, 673 K 4 N O 2 nitrogen dioxide + 2 PbO lead oxide + O 2 oxygen. Nitric Oxide: The melting point of nitric oxide is −164 °C, and the boiling point is −152 °C. Adults over 65: Some older adults tend to be more sensitive to . Switzer Department of .Nitric Acid | HNO3 | CID 944 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. Dinitrogen pentoxide is a colourless solid.PMCID: PMC5137977. Melting Point and Boiling Point.) Nitrogen dioxide (dinitrogen tetroxide) NO 2 /N 2 O 4-11. These reactions occur in mucous secretions, nasal tissues, and capillary blood, leading to the production of NO and N2O.Nitric oxide or Nitrogen monoxide is a chemical compound with chemical formula N O. 2 ), the nitrogen oxides that are most relevant for air pollution.
How to Write the formula for Nitrogen Monoxide
Kinetic study of the nitric oxide oxidation between 288 and 323 K, under pressure, focus on the oxygen influence on the reaction rate constant.Nitric acid is the IUPAC name for this compound, but it is also commonly known by its chemical formula HNO 3, or the names spirit of niter and aqua fortis. It contains nitrogen and oxide ions. This entity has been manually annotated by the ChEBI Team. It is a highly corrosive mineral acid. Chemical Datasheet. The molar mass is 30. The molecule can gain or lose one electron to . Synonyms: NO: nitric oxide, nitrogen oxide, nitrogen monoxide, mononitrogen . Its chemical formula is often given as ·N=O. Formula and structure: The nitrogen monoxide chemical formula is NO. A nitrogen oxide which is a free radical, each molecule of which consists of one nitrogen and one oxygen atom. Before taking NO supplements, reach out to a healthcare provider to discuss the benefits and risks. Nitric oxide also known as nitrogen monoxide is a chemical compound. IUPAC Standard InChI: InChI=1S/NO/c1-2. Nitric oxide (NO) should not be confused with nitrous .Nitric oxide (nitrogen monoxide, •NO) has been intensively studied by chemists and physicists for over 200 years and thus there is an extensive database of . It sublimes at 32. Nitric oxide (NO) should . It is an important signaling molecule in the body of mammals, including humans —one of the few gaseous signaling molecules known. Mononitrogen monoxide.nitrous oxide (N2O), one of several oxides of nitrogen, a colourless gas with pleasant, sweetish odour and taste, which when inhaled produces insensibility to pain preceded by mild hysteria, sometimes laughter.006 Da; Monoisotopic mass 29. It is also a toxic air pollutant produced by automobile engines and power plants.Nitric oxide or Nitrogen monoxide is a chemical compound with chemical formula NO. At a temperature of 140 °C nitric oxide (IV) only consists of molecules of NO2, but very dark, almost black.Oxide: Formula: Mp (°C) Bp (°C) Nitrous oxide: N 2 O-90.To write the formula for Nitrogen Monoxide we’ll use the Periodic Table and follow some s. Fukuto, Jennifer Y.Nitric oxide (NO) is a gaseous molecule that has a central role in signaling pathways involved in numerous physiological processes (e. It has a Molecular mass of 30. It is an efficient oxidising agent which is used as a solvent for the production of many chemical substances. IUPAC Standard InChIKey:MWUXSHHQAYIFBG-UHFFFAOYSA-N Copy. [1] [2] These gases .Nitrogen Dioxide can be made by heating lead nitrate to approximately 673 K. The other two products are water and orange brown nitrogen dioxide gas. IUPAC Standard InChI:InChI=1S/NO/c1-2 Copy.Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i. Nitric oxide is also a heteronuclear diatomic molecule, a class of molecules whose study spawned early modern theories of chemical . An official website of the United States government .Nitrogen Monoxide (Nitric Oxide) Nitric oxide is a colourless paramagnetic gas. Nitric oxide is also a heteronuclear .
Nitric oxide
Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure . It can react with a mixture of air and water to make . Nitric oxide is also a heteronuclear diatomic molecule, a class of molecules whose study spawned early modern theories of chemical bonding.
Biochemistry of Nitric Oxide
It is a colorless gas. Nitrous oxide contains 2 covalent bonds, where one nitrogen is double-bonded to an oxygen atom and triple bonded to another nitrogen atom. Nitric Oxide: The molar mass of nitric oxide is 30 g/mol. Chemical Identifiers | Hazards | Response Recommendations | Physical Properties | Regulatory . Dinitrogen Pentoxide. Its reaction include: Oxidation to NO 2 by oxygen or to nitric acid by permanganate (used for its analysis).
Oxides of Nitrogen: Preparation, Structure, Properties and Uses
Cho, and Chris H.

Substance name: Oxides of nitrogen.In this video we'll write the correct formula for Nitrogen Monoxide.8: Dinitrogen trioxide: N 2 O 3-100. This compound has been prepared and .997990 Da; ChemSpider ID 127983 6-24 hours after exposure, labored breathing and unconsciousness may result.Nitric oxide (NO) a free radical having both cytoprotective as well as tumor promoting agent is formed from l -arginine by converting it to l -citrulline via nitric oxide . These gases contribute to the formation of smog and acid rain, as well as affecting tropospheric ozone., vasodilation, .Nitric oxide (Figure \(\PageIndex{6}\)b) is electronically equivalent to dinitrogen (N 2) plus an electron, and as a consequence it is paramagnetic with one unpaired electron.

NO x gases are usually produced from the reaction between nitrogen and oxygen during combustion of . Nitrous Oxide: The molar mass of nitrous oxide is 44 g/mol.Its chemical formula is often given as ·N=O.Dinitrogen pentoxide, N 2 O 5, is obtained when concentrated nitric acid is carefully dehydrated with phosphorus pentoxide at low temperatures.Chemical formula of nitrogen oxide (IV) NO2.












