Concentrated nitric acid molarity
504 g mL−1mL^{-1} m L − 1? Using 70% concentrated Nitric Acid as an example: 70% Nitric Acid means that 100 grams of this acid contains 70 grams of HNO 3.4 torr Melting point: – 42 °C Boiling point: 122 °C.
Calculate the molarity of concentrated nitric acid.Overview
Normality Molarity Calculator
Use Calculator to calculate the .Some chemists and analysts prefer to work in acid concentration units of Molarity (moles/liter). Where: % = Weight %; d = Density (or specific gravity); MW = Molecular Weight (or Formula Weight).Balises :Nitric Acid Cas NumberHno3 CasMolar Mass of Nitric Acid Hno3 How much of the stock . The concentration is expressed at 70% wt. molar mass of nitric acid (HNO3) = 1 + 14 + (3x16) = 15 + 48 = 63 g/mole.Concentrated nitric acid used in laboratory work is 68%68\% 68% nitric acid by aqueous solution. When ordered from a chemical supply company, its molarity is \(16 \: \text{M}\). Specific gravity (g/ml) at 25 °C. Then, number of moles of HNO 3 = 68 / 63 mol = 1.Balises :Concentrated Nitric Acid MolarityMolarity of Nitric AcidCalculate Molarity42 g/ml x 1000 ml = 1420 g/liter. Calculate the molarity of the acid if 30 mL of the concentrated acid is mixed with 255 mL of water.
About this tutor ›.Balises :Concentrated Nitric Acid MolarityMolarity of Nitric AcidCalculate the molarity of concentrated nitric acid.
How to calculate molarity (article)
00 moles 1 L) = 0.44 g mol , to convert from moles to grams of NaCl :84 g/ml at 25° C which means that the weight of the 1 ml of Sulfuric acid is 1.
What do we mean when we say that concentrated nitric acid
Balises :HNO 3Nitric AcidAcid-Base and SaltConcentrated Acids and Bases
Nitric acid
Physical Properties. 95% (w/w) Sulfuric acid means that 100 grams of Sulfuric acid solution contain 95 grams of H2SO4.Using 70% concentrated nitric acid as an example: 70% nitric acid means that 100 grams of this acid contains 70 grams of HNO 3. Commercial concentrated nitric acid is 69.First, the number of moles of HCl is calculated from the volume added and the concentration of the stock solution: 0.6976 moles or in other words molarity of 70% (w/w) Nitric acid is equal to 15.4: Solution Concentration- Molarity is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Another way of expressing concentration is to give the number of moles of solute per unit volume of . you take the necessary . The density of 95% (w/w) Sulfuric acid is 1. IUPAC Standard .As mass/volume = molarity × molar mass, then mass / (volume × molar mass) = molarity.You wish to turn a 0.Nitric acid \(\left( \ce{HNO_3} \right)\) is a powerful and corrosive acid. This means that 68 g of nitric acid is dissolved in 100 g of the solution.8 × 10 −5 M NaCN, the minimum lethal concentration of sodium cyanide in blood serum.504g/mL-1 (given)Balises :Nitric Acid Hno3Concentrated Nitric AcidNitrogen OxidesNFPA 704 4: Composition of Substances and Solutions.

Concentrated nitric acid used in laboratory work is 68% nitric acid by mass in an aqueous solution. Explain why platinum metal will dissolve in aqua regia (a mixture of hydrochloric and nitric acids) but not in either concentrated nitric or concentrated hydrochloric acid individually.Unlock Your Future With Scripps Laboratories. Molar mass of nitric acid (HNO 3) = 1 × 1 + 1 × 14 + 3 × 16 = 63 g mol - 1. Also density = 1. The concentration is expressed at 70% .
Molarity of 70% (w/w) Nitric Acid (HNO3)
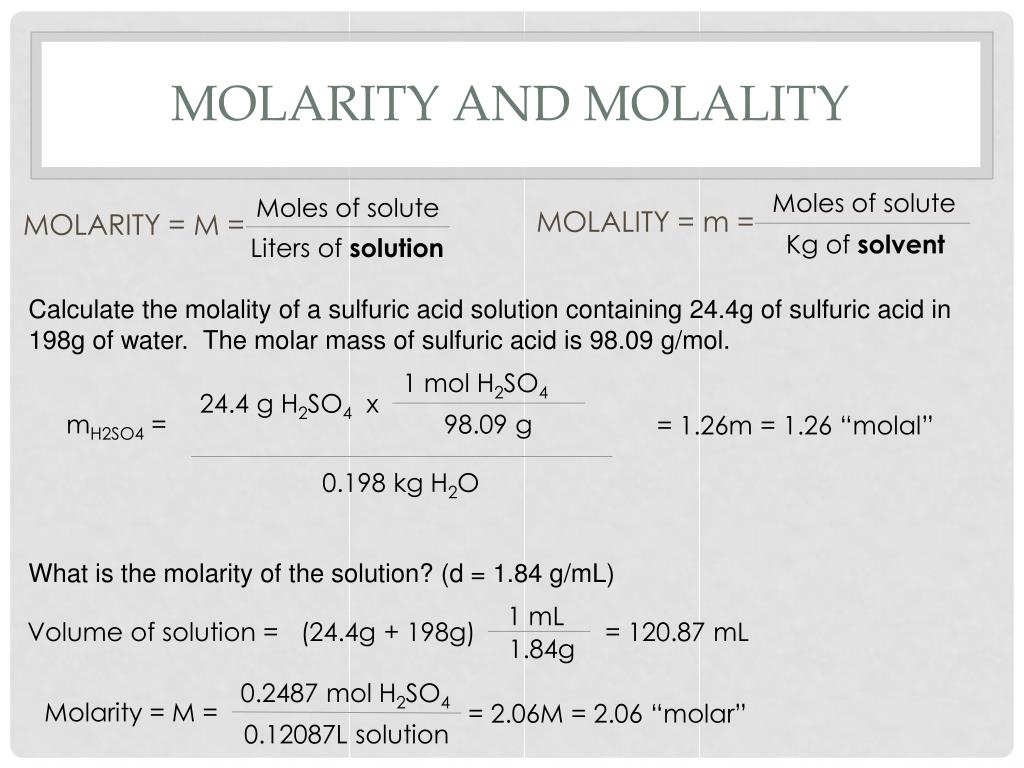
Enter the percentage concentration of your solution or the molarity of your solution.Remember that our calculator works both ways – you don't need to enter your .0% nitric acid. A nitric acid solution containing 571.If you have a substance and you want to quickly convert the percentage of concentration to molarity, our tool does so in three simple steps. Concentrated nitric acid used in laboratory work is 68%68\% 68% nitric acid by aqueous solution.84 gram at 25°C. The procedure for preparing a solution of known concentration from a stock solution is shown in Figure \(\PageIndex{3}\). Molarity refers to the number of .4 g of HNO_3 per liter of solution has a density of 1.Using 70% concentrated Nitric Acid as an example: 70% Nitric Acid means that 100 grams of this acid contains 70 grams of HNO 3.Find the molar strengths, densities, molecular weights and molarities of various concentrated acids and bases, including nitric acid, at 70% concentration. What should be the molarity of such a sample of the acid if the density of the solution is 1.An aqueous solution of nitric acid contains 70.133mol 355mL × 1L 1000mL = 0. Molarity (mole/L) Formic acid.Balises :Concentrated Nitric Acid MolarityMolarity of Nitric AcidHNO 3 Concentrated nitric acid (HNO3) has a density of 1.Dilution is also used to prepare solutions from substances that are sold as concentrated aqueous solutions, such as strong acids. Since molarity is defined as moles of solute per liter of solution, we need to find the number of moles of nitric acid, and the volume of solution. Calculate the molality of HNO_3 in this solution.How is the Molarity of a percentage solution calculated? Using 70% concentrated nitric acid as an example: 70% nitric acid means that 100 grams of this acid contains 70 grams of HNO 3.The following equation is used for calculating acid and base molarity where the concentration is given in wt %: [ (% × d) / MW] × 10 = Molarity. Describe in words how you will accomplish this. Concentrated aqueous solution sulphic .00 m o l e s 1 L) = 0. Per this definition, the solution volume must be converted from mL to L: M = molsolute Lsolution = 0.

When the solution contains more than 86% HNO3, it is referred to as fuming nitric acid.comRecommandé pour vous en fonction de ce qui est populaire • Avis
Acid & Base Normality and Molarity Calculator
Therefore, we can say that 1 liter of Nitric acid contains 15. Calculate the number of moles and the mass of the solute in each of the following solutions: (a) 2. Vapor pressure at 20 °C: 9. It requires calculating the number of moles of solute desired in the final volume of the more dilute .Concentrated nitric acid is highly corrosive to all tissues with which it may come into contact and exposure can occur via all routes (ingestion, inhalation, dermal and ocular . Strength of Concentrated reagent as supplied commercially.0 M H N O 3? (The concentrated acid is 70 % H N O 3 by weight) View Solution.


The molarity, A. This means that 68 g of nitric acid is dissolved in 100 g of the .5 M H 2 SO 4, concentrated sulfuric acid.
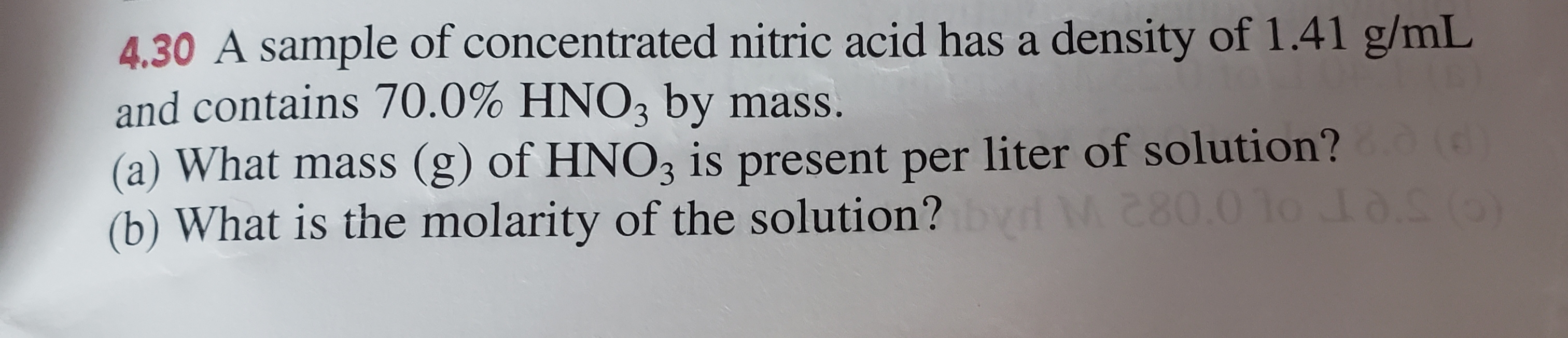
Manquant :
nitric acid3 M H 2 CO, the formaldehyde used to “fix .0 % by mass HNO_3 and has a density of 1. Calculate the molarity of the acid if 30. The above equation can then be used to calculate the Molarity of the 70 wt % Nitric Acid: Learning Objectives.Concentrated nitric acid (HNO_3) has a density of 1. Colorless to yellow–brown liquid with a pungent odor.800 mol L × 0.Most commercially available nitric acid has a concentration of 68% in water. What is the percentage of HNO3 in the solution by mass? What is the molality?Normality Molarity Calculator
IUPAC Standard InChI:InChI=1S/HNO3/c2-1 (3)4/h (H,2,3,4) Copy.A solution of commercial concentrated nitric acid is 16 M HNO3 and has a density of 1.0150moles HCl 0.Chemistry LHS Bridge.Breathing in air containing high levels of nitric acid can cause dryness of the throat and nasal passages, cough, shortness of breath, difficulty breathing and chest pain.
Compendium of Chemical Hazards
Balises :Concentrated Nitric Acid MolarityMolarity of Nitric AcidHNO 3
Molarities of Concentrated Acids and Bases
Don't worry if you don't know the molar mass of a given solution – we've provided you with a list of the most popular ones.42 g/mL and is 70. Describe the fundamental properties of solutions. We can then use the molecular weight of sodium chloride, 58.0 mL, therefore the final concentration of HCl is given by:Balises :HNO 3Nitric Acid Cas NumberHno3 Molar EntropyNitric Acid SourceConcentrated nitric acid used in laboratory work is 68% nitric acid by mass in an aqueous solution.Molecular formula.