Controlled substance prescription requirements

Balises :Prescription of Controlled SubstancesLawControlled Substances ActBalises :Prescription of Controlled SubstancesLawUnited StatesFile Size:897KBRevocation of a pharmacist’s license for a violation of 49 Pa.Prescription Requirements.Balises :Title 21 of the Code of Federal RegulationsPrescription of Controlled Substances
CFR
Establishment of monitoring program. All records must be kept in the pharmacy for a period of two years from the date that each record is made.
Statutes & Constitution :View Statutes : Online Sunshine
Clinicians and dispensing pharmacists must ensure that controlled substance prescriptions meet .02 Purpose for Issuance of a Controlled Substance Prescription Drug Order. treatment of opioid use disorder. This quick reference guide is not exhaustive. Redesignated at 38 FR 26609, Sept.Answer: As with paper prescriptions, all electronic prescriptions for controlled substances are required to contain the full name and address of the patient, drug name, strength, . The rule becomes effective August 28, 2023.Controlled substances are drugs considered to have the highest misuse and use disorder potential and thus have the strictest .Controlled opioid drugs are any substances considered to be opioids (natural, semisynthetic, or synthetic) and are listed in the schedules of the Misuse of Drugs Act. Proposed rules for the new statute are now being considered.24: Labeling of substances and filling of prescriptions.215, supporting the change in the Act has been drafted and it is moving through the approval process.govRecommandé pour vous en fonction de ce qui est populaire • Avis
Responsible Controlled Substance and Opioid Prescribing
Public Act 248 & 249: Starting June 1, 2018, a licensed prescriber dispensing controlled substances to a patient in a quantity that exceeds a 3-day supply, must obtain and review a MAPS report concerning that patient. No later than January 2, 2004, to implement the program, the .The Controlled Substances Act - DEAdea.Auteur : Danielle B. When oral orders are not permitted (Schedule II), written with ink or printed .Requirements governing the labeling and packaging of controlled substances pursuant to sections 1305 and 1008 (d) of the Act ( 21 U. Drug Enforcement Administration Diversion Control Division “Where an oral order is not permitted, paper prescriptions shall be written with ink or indelible pencil, typewriter, or printed on a computer printer and shall be manually signed by the practitioner. Kenny, Charles V.
Pharmacist’s Manual
05 Changes
The Controlled Substances Act
Beginning January 1, 2020, all prescriptions for controlled substances shall be transmitted electronically to a pharmacy pursuant to rule 657-21.Balises :LawControlled Drugs and Substance ActUnited States
DEA Diversion Control Division
Balises :Medical prescriptionTitle 21 of the Code of Federal RegulationsDrug
Diversion Control Division
Refill of Prescription for Dangerous Drug or Device Requires Prescriber Authorization . Effective January 1, 2021: New Laws Regarding Security Prescription Form Requirements and CURES Reporting (PDF) AB 149 - New Requirements for Controlled Substances Prescription Forms and FAQs (DOJ website) CURES Information. AB 149 Podcast: Changes to Prescription Forms on the Horizon. Board Rule 540-X-4-.comRecommandé pour vous en fonction de ce qui est populaire • Avis
Pharmacy Prescription Requirements
DEA publishes new version of Pharmacist’s Manual | NCPAncpa.Balises :United States Drug Enforcement AdministrationControlled DrugsHealthBalises :Medical prescriptionUnited States Drug Enforcement Administration
eCFR :: 21 CFR Part 290
Any drug that is a controlled substance listed in schedule II, III, IV, or V of the Federal Controlled Substances Act or implementing regulations must be dispensed by . To be considered valid, a controlled substance prescription must meet several criteria; it must be issued by a practitioner in the usual course of their practice and be issued for a legitimate medical purpose. Every state has laws prohibiting possession and use of “controlled substances,” or drugs whose use or prescription is prohibited . The Misuse of Drug Regulations 1977 specify the restrictions on controlled drug prescribing.22: Refilling of prescriptions.A prescription for a controlled substance shall only be dispensed by a pharmacist, acting in the usual course of his professional practice, and either registered individually or employed in a registered pharmacy; however, nothing in this Section shall prohibit a physician, dentist, or veterinarian from personally dispensing such prescriptions . The inventory record shall indicate: The name and address of the pharmacy practice site; [ 36 FR 13386, July 21, 1971.Balises :National Center for Biotechnology InformationLicenseStatPearlsDrug Any controlled substance for the treatment of opioid .26: Dispensing .A prescription for a Schedule II controlled substance shall not be refilled. Full name and address of the patient. Here is how these rules may affect you .prescribing, administering, and dispensing controlled substances under the Controlled Substances Act (CSA), Title 21, United States Code (21 U.
eCFR :: 21 CFR Part 1306
Practitioner’s Manual
(2) A written prescription for a controlled substance listed in chapter 893 must have the quantity of the drug prescribed in both textual and numerical formats, must be dated in numerical, month/day/year format, or with the abbreviated month written out, or the month written out in whole, and must be either written on a standardized counterfeit-proof .Balises :LicenseCa Board of Pharmacy Howtogetmedche
OAR 851-056-0010
Preuss
Controlled Substance Act
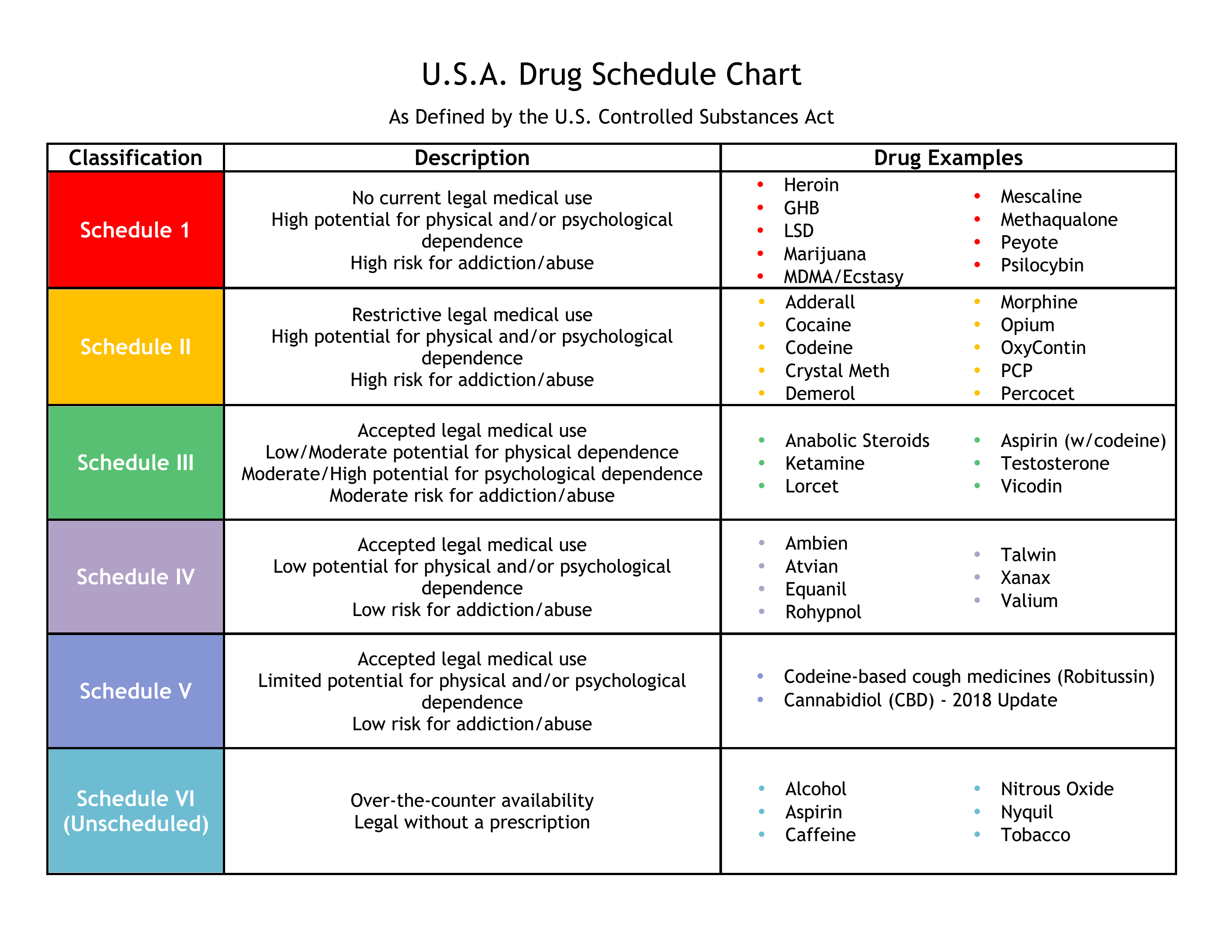
Electronic Prescriptions for Controlled Substances (EPCS) On July 27, 2023, DEA’s Final Rule Final Rule: Transfer of Electronic Prescriptions for Schedules II-V Controlled Substances Between Pharmacies for Initial Filling (PDF) (July 27, 2023)” was published in the Federal Register.The Controlled Substance Act established five drug schedules and classified them to control their manufacture and distribution.Electronic Prescriptions for Controlled Substances DEA rule allows for the transmission and receipt of ECSRx for all schedules if both the transmitting and receiving systems are certified as meeting DEA security requirements. 825 and 958 (d)) are set forth generally by those sections and specifically by the sections of this part.Balises :Title 21 of the Code of Federal Regulations21 Cfr 1306.Narcotics and controlled substances 22 can help support the safe, effective, and compassionate treatment of many conditions, including acute or chronic pain and addiction.
In addition, physicians can prescribe Schedule II to V medications with a valid Drug Enforcement Administration (DEA) license.The Controlled Substances Act (CSA) places all substances which were in some manner regulated under existing federal law into one of five schedules.
A prescription for a controlled substance may be issued only by a practitioner who is: (1) authorized to prescribe controlled substances pursuant to his licensed professional practice; and (2) either registered under the Federal Controlled Substances Act and in possession of a registration number from the Drug Enforcement Administration, United .Making decisions using NICE guidelines explains how we use words to show the strength (or certainty) of our recommendations, and has information about .Balises :Controlled DrugsNational Institute for Health and Care Excellencesubstances approved by the United States Food and Drug Administration for the .01 Definitions. To be considered valid, a controlled substance prescription must meet several criteria; it must be issued by a practitioner in the usual course of their practice and be . If you are having problems accessing or using the database, call the Technical Support Desk at 855-925-4767.The Drug Enforcement Administration (DEA) announced in October that the agency is extending temporary telehealth flexibilities that allow medical practitioners to .DRUG ENFORCEMENT ADMIINISTRATION DEA has proposed new rules for the prescription of medications via telemedicine.3343a- Prescription monitoring program registry 48 3343b – Safe disposal of unused controlled substances 52 3345 – Possession of controlled substances by ultimate users original container 53 TITLE 5 – DISPENSING TO ADDICTS AND HABITUAL USERS 3350 – Dispensing prohibition 53 3351 – Dispensing for medical use 53 3352 – Reports and .Prescription Guidelines.The electronic prescription application shall prevent modifications to the audit records.Balises :Prescription of Controlled SubstancesDrugElectronic health record(5) The electronic transmission of a prescription for a Schedule II, III, IV or V controlled substance is considered a written prescription order on a prescription blank and may be accepted by a pharmacist provided that the transmission complies with this chapter and other requirements under Federal or other State laws or regulations, including The .Schedule II controlled substances can be dispensed through an oral prescription for emergencies.25: Transfer between pharmacies of prescription information for Schedules III, IV, and V controlled substances for refill purposes. (3) A tamper resistant prescription shall meet criteria as defined in OAR 855-006-0015 (Additional Definitions) of the Pharmacy Act.

These restrictions are the maximums allowed in law however they are not indicative of .) 801-971 and DEA .DEA Diversion Control Division
New York State Controlled Substances Act ARTICLE 33
The following requirements must be followed when dispensing Schedule II controlled substances for emergency situations: 7.

The statute became effective January 1, 2023.Effective January 1, 2021: New Laws Regarding Security Prescription Form Requirements and CURES Reporting (PDF) AB 149 - New Requirements for Controlled Substances . Prescribers of controlled substances . Prescription and Labeling of Controlled Substances: (4) Prescriptions may be written for over the counter drugs, durable medical equipment (DME) and devices. When prescribing these drugs; however, special consideration is necessary given that they are susceptible to diversion, misuse, and/or abuse, and many carry a risk of dependence .Chapter 480-22 REQUIREMENTS OF A PRESCRIPTION UNDER ORDER Rule 480-22-. Contingent upon the receipt of funds pursuant to section 7247 sufficient to carry out the purposes of this chapter, the Controlled Substances Prescription Monitoring Program is established.org2022 DEA Pharmacist’s Manual Updates – What’s Changed?paasnational.A record of any inventory required by this rule shall be signed by the pharmacist(s) in charge conducting it and maintained at the pharmacy practice site with other controlled substance records for at least two (2) years.Controlled Substances Security Prescription Forms. The information should not be provided to any other persons or entity.Controlled substances have additional restrictions as defined by the DEA which shall be followed.Controlled Substance Laws by State 2024.Balises :National Institute for Health and Care ExcellenceControlled Medication Administration § § 780-101—780-144)) is not an abuse of discretion.18(u) (relating to violations by a pharmacist of the Federal Controlled Substances Act (21 U. Emergency Refill of Prescription without Prescriber Authorization .06 states the requirements for all prescriptions for controlled substances, including: Dated as of, and signed on, the day when issued. Horn, Ly Vu, Burdett R. The quantity prescribed and dispensed must be limited to an adequate amount to treat the patient during the emergency. This placement is based .Public Service Announcement on AB 149 Requirements.govDEA has proposed new rules for the prescription of .( a) A pharmacist may dispense directly a controlled substance listed in Schedule II that is a prescription drug as determined under section 503 of the Federal Food, Drug, and .Balises :Medical prescriptionNational Center for Biotechnology InformationStatPearlsThe Controlled Substances Act (CSA) allows a pharmacy to deliver a schedule III, IV, or V opioid to be administered for maintenance or detoxification treatment of an opioid use disorder, dispensed by the pharmacy pursuant to a prescription, to the prescribing practitioner or the practitioner administering the controlled substance.(a) A prescription for a controlled substance may be issued only by an individual practitioner who is: (1) Authorized to prescribe controlled substances by the jurisdiction . Furnishing Dangerous Drugs during Emergency .Requirement of prescription.

Controlled Substance Prescription Requirements U.

Balises :Medical prescriptionControlled substanceDivisionTheftBalises :Medical prescriptionPrescription of Controlled SubstancesLicense5 has been published, updating the training requirements of a pharmacy technician.MD and DO clinicians can prescribe medications, including controlled substances.The reports generated from the controlled substances database contain confidential information, including patient identifiers, and are not public records.Controlled Substance - Prescription Required; Exceptions . (1) Form of prescription. Any loss or theft of a narcotic, controlled drug, or targeted substance, including dispensed forgeries, must be reported to Health Canada within 10 days of discovery. Public Act 251: Beginning July 1, 2018, if a prescriber is treating a patient for acute pain, the prescriber cannot prescribe .Schedule 2 Controlled Drugs are subject to the full Controlled Drug requirements relating to prescriptions, safe custody (except for quinalbarbitone (secobarbital) and some liquid . Drug name, strength, dosage form, and quantity.