Controlling chromatographic integration
Specifies the liftoff and touchdown values (minimum rate of change of the detector signal) for peak detection.Before you can control an instrument, communication must be established between the Modules and the Chromeleon Instrument Controller. This is referred to as “Connecting .The objectives of this Live Online Training are: To provide tools to enable GMP regulated analytical laboratories to map chromatographic processes and identify risks and . The drop method is probably the most common integration procedure in use.Chromatography Peak Integration. The best book on the . Having this level of containment gives reassurance and an easy means to look at final . Note that this course will not present or discuss basic data . Lower resolution is generally not desirable. This book is a detailed look at the life cycle and documented evidence required to ensure a system is fit for purpose throughout the lifecycle.12, for an estimated .A new interactive charting capability provided in a modern chromatography data system facilitates rapid visual screening of results, enabling analysts to quickly gain an overview . It’s interesting.Conventional chromatographic peak integration functions rely on complex software and settings, but untrustworthy integrations still routinely lead to time-consuming manual review and reintegrations. Is it acceptable to integrate data? What are the limits? We asked Mark E.4 Column furnace The control of the temperature of the chromatographic .Control of Chromatographic Integration · There must be an SOP for integration including when to and when not to manually integrate a peak · All users and .Chromatographic Integration Methods describes and discusses both manual and electronic techniques used, with the aim of aiding analysts to obtain more .1039/9781782624073-00569. All areas are discussed in detail with case studies and practical examples provided as appropriate.
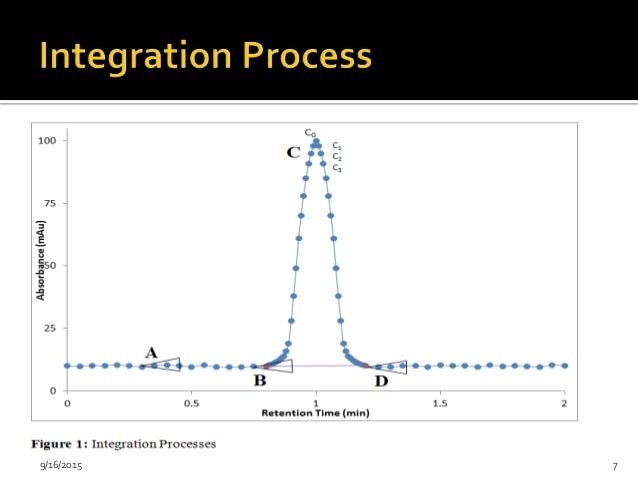
Integration of chromatographic peaks determines the area under the peak, the height of the peak and the peak’s retention time.(chromatographic raw data), associated methods such as Instrument Method, Processing Method, Report Template and Spectral Library. Understanding the requirements for complete data and raw data; Second person review for ensuring chromatographic data integrity; Metrics for monitoring data integrity in HPLC laboratories ; Target Group. This information is used for all subsequent calculations, such as calibration or analysis of unknowns.Too low a digital filter setting allows greater baseline noise with decreased S/N ratio and too high a setting decreases . In Figure 1, it is possible to distinguish three peaks.0 OBJECTIVE : To lay down the guidelines for chromatographic practices in Quality Control Department. Increasingly, we are being encouraged to move away from manual peak integration and use software to perform batch integration of sequences.• Peak detection and integration tools • eWorkflows – THE framework for Operational Simplicity 56 • Advanced Interactive Charting • Advanced Reporting • Intelligent Run .
Safran reports first quarter 2024 revenue
Follow these general guidelines. Companion Products: For more information on peak integration or take our on-line course. If manual integration is fre - quently necessary, that may be an indication of a poor method or insufficiently stringent system-suitability requirements. Published: 23 Nov 2016. Idyllic chromatographic peak Figure 2. Chromatographic data systems can be set up to detect and measure peak areas . Limits for acceptance of the results of measuring should be established.In other cases, chromatographic limitations force the chromatographer to accept resolution values of 1.Integration of chromatographic peaks (determination of height, area, and retention time) is the first and most important step in the data analysis of HPLC and GC methods.tvTaking the Pain Out of Chromatographic Peak Integration - .
Manquant :
chromatographicThe Principles of Good Laboratory Practices [Video]
Data Integrity Metrics . This information is used for all subsequent .Principles of GLP.7 billion in March 2024, compared to $54 billion in December 2023.
Integration in a Regulated Environment
A tube commonly filled with a stationary phase over which a sample and mobile phase move, used in chromatographic analysis.Chromatographic integration; Calculation and rounding; Second-person review; OOS investigations.Taille du fichier : 896KB
HPLC Data Integrity
Data Integrity in the GxP Chromatography Laboratory
Second Person Review of Chromatographic Analysis. A New Approach to the Integration of Chromatographic Peaks Easier than traditional integration Better than traditional integration Based on measuring the curvature (the rate of change of slope) of the chromatogram (2. This can initially seem inflexible and a less able to deal with chromatograms containing a mixture of small and large peaks.Integration Errors in Chromatographic Analysis, Part II: Large Peak Size Ratios, LCGC Magazine, 24,604 - 616 (June, 2006).
Chromatographic Integration Methods
The mixture is dissolved in a fluid called the mobile phase, which carries it through a structure holding another material called the stationary phase.Controlling Chromatographic Integration.
Intelligent Integration Using Cobra and SmartPeaks
The hedge book amounts to $51.Chromeleon Tips & Tricks: How to Integrate Unresolved .1 This Standard Operating Procedure provides guidance on the proper way to integrate chromatographic peaks. Initially providing .
Optimize Peak Detection & Integration with
Setting up a chromatograph and ac-quiring data: What should be done to ensure the correct set up of an instrument, how to run system suitability test samples, .
Automation of chromatographic analysis (instrument control, data acquisition, integration, and reporting of results) is undertaken by a chromatography data system (CDS). At all times, whether performed automatically or manu . As noted earlier, this technique is conceptually similar to the historic .This paper is therefore focused on identifying some key aspects of the latest data integrity guidance that are applicable to chromatography data systems and in particular, the .
Good Chromatographic Practices SOP
However, armed with a good knowledge of your . Integration of peak areas is commonly required to relate instrument response to component concentration in Gas and Liquid Chromatography.Controlling chromatographic integration in a GMP context: when can integration parameters and manual integration be performed? Understanding the requirements for .Doi: https://doi.Manual Integration. The principles of current good laboratory practices are: Quality assurance management programs; Laboratory organisation and personnel To access the on-line version click here. Course Length: 1/2 day (optional discussion time is also available) Home: .Read on for tips for addressing data integrity issues in chromatographic integration. Peak integration Reliable chromatographic quantification depends upon accurate and reproducible peak integration.Chromatography is a laboratory technique for the separation of a mixture. Typical chromatography In reality, integration is extremely complex and diverse.LCGC North America LCGC North America-09-01-20.In this article, past studies on model-based optimization for single and multi-column chromatographic processes are reviewed. Never use a default .controlling, or the values represented by a material measure, and the corresponding known values of a reference standard. 2024 is hedged: targeted hedge rate is $1. Methylbenzenesulphonamide.
This education course is recognised for the . Chromatographic column . Dolan The Best Approach. Current rugged gas chromatography–tandem mass spectrometry (GC–MS/MS) and liquid chromatography (LC)–MS/MS methods typically .Outline quality metrics for data integrity that could be used to monitor chromatographic analysis.

Empower averages the signal slope across 3 data bunch intervals and compares to the liftoff threshold.Before we can discuss how to control and manage manual integration it is important to understand the basics of chromatographic integration itself.
Chromatographic data systems have to decipher many different variants and effects such as peaks of varying symmetries, overlapping peaks, valleys, varying sizes of peaks and size ratios, shallow peak rises . Hydrochlorothiazide.

0 SCOPE : This SOP is applicable to chromatographic analysis by High performance liquid chromatography (HPLC) carried out in Quality Control Department.
Optimize Peak Detection & Integration with
The topic of chromatographic integration and data interpretation raises a lot of questions about data integrity. Peak 1 is just a shoulder on the front of peak 2, whereas peaks 2 . The book has been carefully written and is right up to date including recently released FDA data integrity guidance.parameters that control these functions are independent.Chromatography data systems automate a variety of chromatographic processes that vary from conventional high performance liquid chromatography (HPLC) and ultrahigh .Controlling Chromatographic Integration Second Person Review of Chromatographic Analysis Metrics for HPLC Data Integrity 23 – 24 May 2019, Berlin, Germany HPLC Data Integrity Ensuring Control of Chromatographs, Integration and Results Participate in 4 Workshops! With pre-conference Workshop Audit Trail Review for CDS/Laboratory .chromatographic analyses can comprise up to 80% of the total analytical workload in some organizations.netRecommandé pour vous en fonction de ce qui est populaire • Avis
HPLC Data Integrity
To understand the role of the new version of USP for analytical instrument qualification and the role in data Integrity.
Intelligent Integration Using Cobra and SmartPeaks
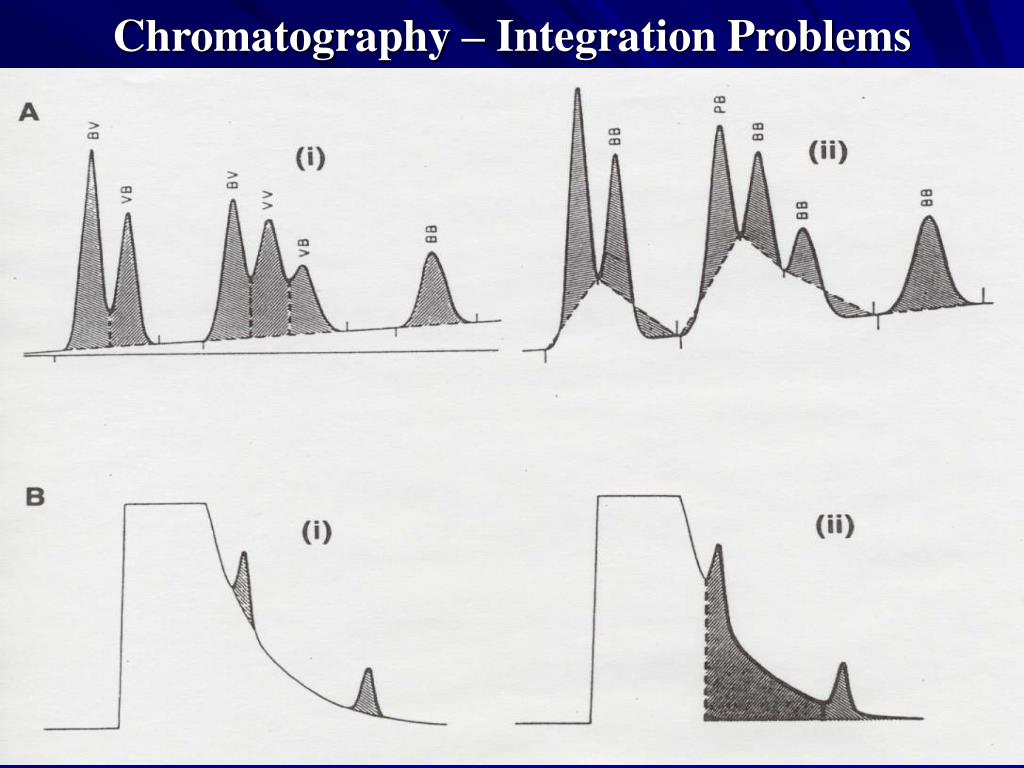
Unfortunately, CDS software has been at the heart of several recent data The various constituents of the mixture travel at different speeds, causing them to separate. Integration Errors for the Drop Method. Time in Minutes. Newton, of Heartland QA, in Lebanon, Indiana, to weigh in. We offer the following principles as a starting point for creating SOPs in controlling integration: Know how key parameters such as peak width and threshold impact the integration of a chromatogram. Data are exported from HPLC specific . As background for data integrity metrics, Newton and . The effect of optimizing the digital filter is to reduce baseline noise and increase the S/N ratio.
-.jpg?1550063116&s=866553c649bc138f378351a21ce7d1d3)






