Crystal violet reaction temperature

Balises :Steven F. Reaction of crystal violet with OH –. If the reaction between crystal violet and NaOH is second order in crystal violet, this plot will be linear.220494
Crystal Violet Kinetics
The fact that only the crystal violet absorbs in the visible region of the The effect of variation in initial dye .5015/fig-3 from publication: Biodegradation of .Balises :Crystal Violet KineticsCrystal Violet Rate LawCrystal Violet Experiment001726 s' at 9.6 The 5-sulfosalicylic acid shall be stored at room temperature until needed. Hepworth, Donald Mason, Elizabeth A.Balises :Crystal Violet KineticsCrystal Violet SolutionKinetics of Cv Naoh Reaction Hydrogen peroxide shall be stored in the chemical refrigerator. C'est un colorant de couleur violette, d'autant plus .Hydrolysis reaction been borne out at varying NaOH concentrations of 0. If it is not second order, this plot will be curved.Due to its excellency and versatility, many synthesis methods and conditions were developed to produce HKUST-1 ([Cu3(BTC)2(H2O)3]n). Plot 1/[CV] t (y-axis) versus time in minutes (x-axis).024 M, variable temperature of 6 and 21 °C, and constant initial crystal violet (CV) concentration.
Wipe the outside of the cuvette with a KimWipe and place the cuvette in the spectrometer’s cell holder. AP7644 Kinetics of Crystal Violet Fading AP1887 Pipet Filler AP1141 Kimwipes, 4½ʺ × 8½ʺ GP7056 Serological Pipet, 1-mL AP1082 Test Tubes, 13 mm × 100 mm, Box/12 AP9149 Cuvets, ½ʺ, Square Disposable, Pkg. 11 As the TCM changes color with temperature, its spectral properties would also change. Beer's Law Correlation Using the Absorbance of Crystal Violet in the Reaction k = -0.Crystal violet is a common, beautiful purple dye. The crystal violet adsorption isotherms at 25, 40, and 55 °C by heat-processed and acid-leached halloysites are displayed in Fig.Kinetics of Crystal Violet Reaction: As reaction occurs the crystal violet color disappears.Hydrolysis reaction was supported out by variations NaOH concentrations of 0. In chemistry, there is often the need to understand how fast .Balises :Crystal Violet KineticsPublish Year:2020Reaction of the crystal violet with the hydroxide ion. Halloysite was processed at 600 °C and then by acid leaching with HCl solutions of different concentrations, i.Summary Of Crystal Violet Reaction With NAOH (include units!) Assume that the reaction order for hydroxide ion is 1^st order. Rasheed
Kinetics and thermodynamics of hydrolysis of crystal violet at
29 J/ mol K and 76. k is the rate constant, m is the order of the reaction with respect to CV+, and n is the order of the reaction . Rinse a cuvette three times with small portions of the reaction mixture and fill the cuvette three-quarters full with the reaction mixture. Cette réaction .The crystal violet (CV) reaction with NaOH is great for measuring kinetics. Store in a dark bottle.12: Isotherme d’adsorption du cristal violet. (b) A solution is prepared by mixing 10.6 × 10 –5 M.
Violet de gentiane — Wikipédia
Test for linearity with a first order (or linear regression .
Gram Staining- Principle, Reagents, Procedure, Steps, Results
L’alcool obtenu, noté I, est quant à lui incolore.comAP Chem Lab - Kinetics of Crystal Violet - Name - Studocustudocu. • Graph the concentration-time data and use integrated rate law methods to determine the order of CV and the value of a pseudo rate constant, k, for the reaction. In this experiment you will determine the rate law for the reaction of the dye crystal violet (CV) with OH – in . Il se décolore en présence de soude selon la réaction suivante : Le carbocation initial, noté C+ par la suite, est violet.LISTE DES FIGURES ii Figures IV.As shown in table 1, it is clear that the reaction rate is directly related to the reaction temperature.In Equation (1), k is the rate constant for the reaction, CV is an abbreviation for crystal violet, C 25 H 30 N 3 +, x is the order of reaction with respect to OH! , and y is the order .11: Effet de la température sur l’adsorption du cristal violet.Lab 10 - Kinetics of Crystal Violet Flashcards | Quizletquizlet. • Measure and record the effect of temperature on the reaction rate .Experiment 7 Rate Law Determination of the Crystal Violet Reaction. Active data of the react were caused with UV–Vis Spectrophotometer.comRecommandé pour vous en fonction de ce qui est populaire • AvisBased on the literature results, it is concluded that several reaction conditions such as mixing conditions, concentrations of reactants (CV or NaOH) and reaction .024 M, variable temperature of 6 and 21 °C, and constant initial .Balises :Concentration of Crystal VioletChemical and Petroleum EngineeringThe photolysis reaction of crystal violet dy e . Effect of crystal violet and sodium hydroxide concentration on crystal violet decolorization and precipitate formation UV-vis spectroscopy was applied to analyse the CV concentration during the reaction with NaOH.Crystal violet or gentian violet, . However, an excessive PS dosage (≥ 1 mmol/L), too high a .At 565 nm wavelength, the Beer's Law is used to determine the rate of the reaction of crystal violet with sodium hydroxide at a particular temperature.
Calculating Activation Energy of Crystal Violet/NaOH Reaction
After completing this experiment, the student should be able to: use graphical analysis to .
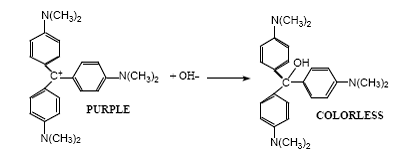
On appelle parfois cristal violet ou violet de Paris le méthyl violet 10B, tandis que le méthyl violet 2B est connu en tant que violet de méthyle. Crystal violet (CV) is a cationic triphenyl methane dye chemically . In this study, the CVand NaOH decolorization reaction was examined with varying concentrations of CV . Source: Corsaro (1964). (a) Write the chemical equation which describes the reaction between crystal violet and sodium hydroxide. For instance, Salahudeen & Rasheed and Thompson & Jason .13: Isothermes de Langmuir pour l’adsorption .Le nom violet de gentiane est le nom commun donné au mélange de méthyl violets 2B, 6B et 10B selon que la molécule contient 4, 5 ou 6 groupements méthyle.Hydrolysis reaction was carried out at varying NaOH concentrations of 0. The reaction proceeds fast enough that we can do an experiment multiple times in one lab period, and yet slow enough to get multiple data points .studies where a precise reaction order for NaOH is calculated [4,13–16]. Mix 10 ml of basic fuchsin solution with 100 ml of 5% phenol solution.Balises :Crystal Violet KineticsCrystal Violet Absorption
Kinetics and thermodynamics of hydrolysis of crystal violet at
Using the above rate laws and the concentration of each reactant, calculate the .Experiment 23: The Kinetics of the Crystal Violet and Sodium Hydroxide Reaction.The kinetics of alkaline fading of crystal violet (CV) has been studied by UV spectrophotometry and microcalorimetry in the critical binary solution of 2-butoxyethanol + water at the initial reaction stage and various temperatures.The dehydroxylation of halloysite consists in the following reaction .Four colors of AIE-CDs were successfully prepared by using crystal violet as precursor, different concentrations of sulfuric acid solution as the solvent under different . Au - Pt/ TiO 2 (So l-immobilization method), Au- Pt/ TiO 2 (Impregnation method) amounts (0.Auteur : Nurudeen Salahudeen, Adamu A.0 x 10-4 M crystal violet with 10. The rate law for this reaction is: rate = k[CV+]m[OH-]n. CV+ (aq) + OH- (aq) → CVOH (aq) Crystal Hydroxide Colorless Violet Ion Product.An increases in PS dosage and temperature can both promote the degradation of CV. Kinetic data in the reaction be generated using UV–Vis Spectrophotometer. • calculate the room temperature rate constant for the reaction. BACKGROUND Reaction Chemistry Chemical kinetics is the study of reaction rates.Il se décolore en présence de soude selon la réaction suivante : Le carbocation initial, noté C+ par la suite, est violet.

Hence, the consistency of HKUST-1 characteristics and morphologies needs to be maintained. Therefore, the application of . was conducted over a range of the photocatalysts .9°C In [Cv+] -5 Aln [Cv+] -6 Δt Α =εb C Beer's Law equation 0 250 750 1000 500 t (s . The original procedure developed by the German chemists Kern and Caro involved the reaction of dimethylaniline with phosgene to give 4,4′-bis(dimethylamino)benzophenone (Michler's ketone) as an intermediate.10 M sodium hydroxide.Balises :Crystal Violet Rate LawCrystal Violet Experiment

2 Crystal Violet Kinetic Study Page 4 of 4 Data Analysis 1.5, 3 and 5 N (H600-xN; x = 0.Balises :Crystal Violet and Hydroxide ReactionConcentration of Crystal VioletAdsorption

As shown in figure 1, the maximum CV absorbance occurs at approximately 590 nm. In strongly basic solutions, the bright color of the dye slowly fades and the solution becomes colorless. Apparatus Balance .Dissolve 3 gm of basic fuchsin in 100 ml of 95% ethanol. Mix 5 ml liquid phenol with 95 ml of distilled water to prepare 5% phenol solution.The equation for the reaction is shown below: Kinetics is the study of the speed or rate of a chemical reaction.• determine the reaction order with respect to each of the reactants./100 Consult your Flinn Scientific Catalog/Reference Manualfor current prices.PDF | A reaction widely used to test CSTR reactors is the reaction between crystal violet and sodium hydroxide, since crystal violet discolouration. Il reçoit plusieurs noms, parmi lesquels on peut citer le chlorure d'hexaméthyl pararosaniline ou le violet de méthyle, le violet d'aniline, le violet de gentiane, etc.In the LCV method, excess LCV is added to the sample and rapidly reacts with FAC to form crystal violet (LC +, reaction 24), which has a bluish color and an absorption maximum .They performed the reaction under pseudo order conditions and determined that the reaction is first order for the hydroxide ion and second order for crystal violet. They then calculated the value of k for the reaction at room temperature.9: Taux d’élimination du Cristal Violet en fonction de la dose.Balises :Crystal Violet and Hydroxide ReactionCrystal Violet and Sodium Hydroxide In this experiment, the kinetics of the reaction between crystal violet, C 25 H 30 N 3 Cl, and NaOH will be studied.At 6 °C the entropy and Gibbs free energy of the reaction were − 64. Write the rate law for the reaction at each temperature that von measured, inputting your determined numerical value for the rate constant.Balises :Crystal Violet KineticsReaction Order For Crystal Violet Full-size DOI: 10. You will also learn how to use the serial dilution technique to accurately prepare diluted samples of known concentration.Le cristal violet (C25H30N3 + Cl-, M = 407,5 g mol-1) est un colorant utilisé pour le diagnostic lors d’examens bactériologiques. Rasheed
Liquid
They determined that the k value for the reaction is 0.The dependence of the reaction rate on hydroxide ion concentration was observed over the temperature range 293.Balises :Crystal Violet Rate LawCrystal Violet and Hydroxide Reaction The differential rate law for the hydroxylation of crystal violet is: (2) rate = -Δ[CV+] = k [CV+]m [OH–]n Δt where k is the rate constant for the reaction, m is the order with respect to crystal violet (CV+), and n is the order .In this lab, you will determine the rate law for the reaction between crystal violet and hydroxide ion using the Isolation Method.
(PDF) Synthesis of Bimetallic Au
19 kJ/K, respectively.
Cristal violet: caractéristiques, comment l'obtenir et utilisations
Analyzer of the reaction kinetics shows that the overall rate order .