Cytochrome p450 biotransformation

Summary This chapter contains sections titled: Introduction Cytochrome P450-Dependent Steroid Hydroxylase Systems Native Microorganisms in Steroid Biotransformation 11α-Hydroxylation 11β-Hydroxylat.
Manquant :
biotransformationLes cytochromes P450
Cytochrome P450 — Wikipédia
Cytochrome P-450 Enzyme System. Structure tridimensionnelle de la NADPH-cytochrome P450 réductase ( PDB . In this study, a recombinant E. Small-scale studies confirmed that both CYP4F2 and CYP4F3A were capable of oxidizing the substrate, with the former . Gregg, in Encyclopedia of Gastroenterology, 2004 Enzymatic Biotransformation of Drugs. In vitro, no metabolism was detected with kidney microsomes, whereas two metabolites were generated by liver microsomes. The determination of antigenic cross-reactivity of algal proteins with heterologous polyclonal antibodies originally raised against plant P450s, anti-cinnamic acid 4-hydroxylase (CYP73A1), anti-ethoxycoumarin-O-dealkylase, anti-tulip .The Stimulatory Role of Human Cytochrome b 5 in the Bioactivation Activities of Human CYP1A2, 2A6 and 2E1: A New Cell Expression System to Study . Cytochrome P450s (CYP) are monooxygenase enzymes that efficiently perform Phase-I biotransformation of xenobiotics including Bisphenols [20], [21], [22]. The time-course biotransformation was characterized by . Phase II: Yields a large polar metabolite by . Les cytochromes P450 (CYP) constituent une superfamille de 57 gènes codant pour des enzymes qui métabolisent un grand nombre de médica-ments mais également . Cytochrome P450 réductase. Given the strong trend to implement zebrafish (Danio rerio) embryos as translational model not only in ecotoxicological, but also toxicological .The self-sufficient cytochrome P450 BM3 mutant (A74G/F87V/D168H/L188Q) can serve as a biocatalyst for whole-cell catalysis process of indigo.Biotransformation of Bisphenol by Human Cytochrome P450 2C9 Enzymes: A Density Functional Theory Study Inorg Chem. The expression of cytochrome P450 and related biotransformation is altered during the operation of host defense mechanisms.
Cytochrome P450 regulation and drug biotransformation during
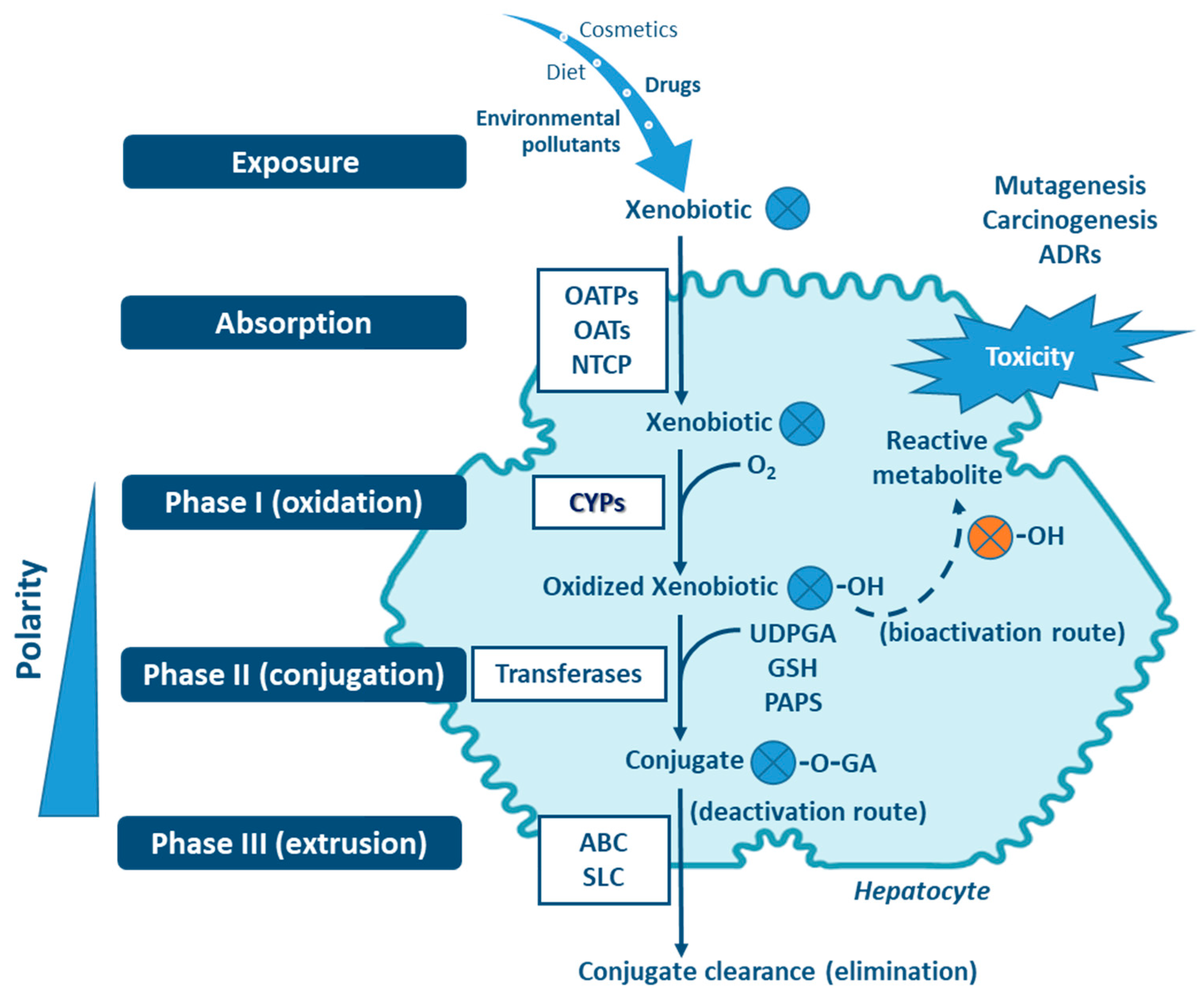
Human Cytochrome P450 (CYP) enzymes constitute a superfamily of membrane-bound hemoproteins that are responsible for the metabolism of a wide variety .The cytochromes P450 (CYPs) constitute the major enzyme family capable of catalyzing the oxidative biotransformation of most drugs and other lipophilic .Le rôle des cytochromes P450 dans les interactions médicamenteuses et environnementales rencontrées à l'officine. The cytochrome P450 system catalyzes the insertion of an oxygen atom into C H and N H bonds, the epoxidation of π bonds, and the addition of an oxygen .NADPH-cytochrome P450 reductase expression and enzymatic activity in primary-like human hepatocytes and HepG2 cells for in vitro biotransformation studies. Les données contenues sur la carte plas-tifiée des CYP jointe à cet article doivent être perçues comme un outil pratique permettant une appréciation . Understanding the CYP . “Xenobiotic biotransformation” refers to the process by which a compound foreign to .This study examined the cytochrome P450 (CYP) enzyme selectivity of in vitro bioactivation of lynestrenol to norethindrone and the further metabolism of norethindrone.Biotransformation of VOCs is catalyzed largely by cytochrome P450 s in liver. CYPs involve porphyrin cation radical iron (IV) oxo species generally known as Cpd I as their primary oxidant for the transformation . Amélie Mathis (1) Afficher plus de détails. Nevertheless, for industrial applications of such enzymes, e.Previous experimental studies have shown that XPs can be catalytically transformed into epoxides and haloquinones by cytochrome P450 enzymes (CYPs). 54–96), Vol 4 of Comprehensive Toxicol.Cytochrome P450-dependent biotransformation in (eco)toxicology. In humans, almost 80% of oxidative metabolism and approximately 50% of the overall elimination of common clinical drugs can be attributed to one or more of the .Since cytochrome P450 system substrates are often insoluble in water, reactions should be carried out at high temperatures and in the presence of organic solvents where the high stability of all of P450cam, PdR, and Pd are needed.The biotransformation of 17-HP was performed in whole cells and the product analysis indicated that MA_CYP17A2 possessed the activities of progesterone C17-hydroxylate (17-HP titer, 43.This study was aimed at identifying the isoform(s) of human liver cytochrome P450 (CYP) involved in the hepatic biotransformation of trans-resveratrol (trans-3,5,4'-trihydroxystilbene).The secondary metabolite pseudopyronine B, isolated from Pseudomonas mosselii P33, was biotransformed by human P450 enzymes, heterologously expressed in the fission yeast Schizosaccharomyces pombe. This enzymatic . Immunoinhibition with anti-P450 3A4 . Cytochrome P450 enzymes are an integral element towards the emergence of ancestral eukaryotes in the primeval prokaryotic era.Experimental approach: As all CYPs depend on cytochrome P450 reductase (POR) as electron donor, we generated a smooth muscle cell-specific, inducible knockout mouse of POR (smcPOR-/-) to investigate the contribution of POR/CYP to vascular biotransformation of organic nitrates. The P450-catalyzed biotransformation of the analgesic drug paracetamol (PAR) is a long-debated topic, involving different mechanistic hypotheses as well as experimental evidence for the metabolites N-acetyl-p-benzoquinone imine (NAPQI), p-benzoquinone, acetamide, and 3-hydroxy-PAR. Trans-resveratrol metabolism was found to yield two major metabolites, piceatannol (3,5,3',4'-tetrahydroxystilbene ., steroid hydroxylation, several challenges .Les cytochromes P450 (ou CYP450) sont des enzymes ubiquitaires (présentes notamment dans le foie, les entérocytes et les surrénales), qui interviennent .Pesticide biotransformation, especially by cytochrome P450 enzymes (CYPs), may produce metabolites with substantially altered toxicological and physicochemical profiles, which has drawn great . For understanding possible toxicity differences to adult fish, it might be crucial to understand the biotransformation of chemicals in zebrafish embryos i. (McQueen CA, Series Ed. More than 30 distinguishable cytochrome P450 enzymes have been described.
Role of Cytochrome P450 Enzymes in Biotransformation
Biotransformations
Perspective: Mechanisms of cytochrome P450-catalyzed .

Cytochrome P450 enzyme-based biotransformation of pharmaceuticals and personal care products (PPCPs) by microalgae in the aquatic environment - .), Biotransformation (3rd ed.Human cytochrome P450 (CYP) enzymes, as membrane-bound hemoproteins, play important roles in the detoxification of drugs, cellular metabolism, and homeostasis. Biotransformation in the zebrafish embryo .Here, we report a multifunctional cytochrome P450 enzyme, PyrE2, which catalyzes the regioselective, successive 6-electron oxidation of an inert methyl group to produce a carboxyl product through formation of the hydroxyl and aldehyde intermediates in pyrroindomycin biosynthesis. The biotransformation . Under initial rate conditions and high substrate concentration (400 microM midazolam), vari . Screening with well-established chemical inhibitors showed that the formation of norethindrone was potently inhibited by CYP3A4 inhi . as part of toxicokinetics.Figure 1 shows the known generalized pathways associated with drug metabolism catalyzed by cytochrome P450 (CYP) enzymes.The capabilities of cytochrome P4503A4 (CYP3A4), CYP3A5, and fetal hepatic microsomes containing CYP3A7 to metabolize midazolam were investigated using human hepatic microsomes and purified CYP3A4 and CYP3A5. Metabolism of BPA and their analogues (substitutes) is generally performed by liver cytochrome P450 enzymes and often leads to a mixture of products, and some of those are toxic.
Biotransformation of Xenobiotics
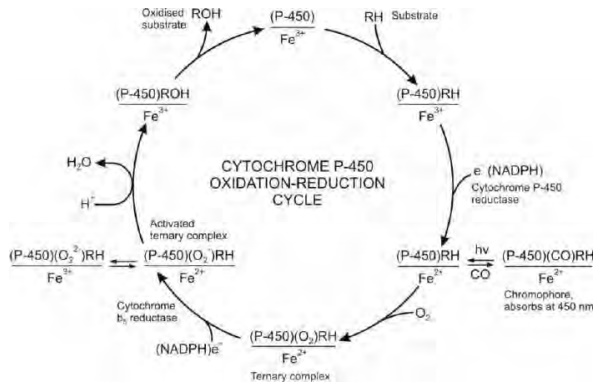
Not much is known about the biotransformation capability of zebrafish (Danio rerio) embryos.The results suggest that the N-demethylation of metflurazon by both algae is mediated by a cytochrome P450 monooxygenase. During the catalytic cycle of P450, a . FK506 and rapamycin metabolism was significantly correlated with nifedipine oxidation in human liver microsomes of eight different individuals.The hepatic cytochrome P-450 responsible for metabolism of the structurally related macrolides FK506 and rapamycin in humans was identified using in vitro studies.Previous studies from our laboratories have shown that the metabolism of the cholesterol-lowering drug lovastatin by rat and human liver microsomes occurs primarily at the 6'-position, giving 6' beta-hydroxy- and 6'-exomethylene-lovastatin and that these oxidations are catalyzed by cytochrome P450-d .Oxidation with cytochrome P450 (most common) Reduction. Oxford, UK: Elsevier.Vue d’ensemble
Biochemistry, Biotransformation
P450 substrate specificity and catalytic efficiency can be readily assessed in vitro, for example, through assays that use recombinant human P450 enzymes. [Google Scholar] Guengerich FP (2018b). Notes et références.Experimental and Computational Studies on the Biotransformation of Pseudopyronines with Human Cytochrome P450 CYP4F2 November 2022 Journal of Natural Products 85(11) Taxol metabolism by human . Nevertheless, the bioconversion yield of indigo is generally low under normal cultivation conditions (37 °C, 250 rpm).Cytochrome P450 (P450 or CYP) enzymes are responsible for the bulk of phase I biotransformation of xenobiotic compounds. Computational insight into biotransformation of halophenols by cytochrome P450: Mechanism and reactivity for epoxidation .Auteur : Francisco Esteves, José Rueff, Michel Kranendonk They are found in highest concentration in the liver and, in the case of certain subfamilies, in the small intestinal mucosal enterocytes and other organs.
Xenobiotic biotransformation in unicellular green algae
The most prominent metabolite, termed M5, .Conclusion and outlook.Precision Biotransformation of Emerging Pollutants by Human Cytochrome P450 Using Computational–Experimental Synergy: A Case Study of Tris(1,3-dichloro-2-propyl) .Cytochromes P450.
However, the role of P450-mediated metabolism in .Cytochrome P450.Publiée : 2023/08/14Human Cytochrome P450 (CYP) enzymes constitute a superfamily of membrane-bound hemoproteins that are responsible for the metabolism of a wide variety of clinically, physiologically, and .Cytochrome P450 (CYP) is a hemeprotein that plays a key role in the metabolism of drugs and other xenobiotics (Estabrook, 2003).Parmi les cytochrome P450 les plus impliqués dans le métabolisme des médicaments, on retrouve par ordre décroissant le CYP3A4 (plus de 50% des médicaments), puis 2D6, 2C9, 1A2 et 2E1.
Manquant :
biotransformationCytochrome P450 réductase — Wikipédia
Moreover, cytochrome P450 oxidoreductase (CPR) was optimized from five different species by co-expressing them in engineered .Since cytochrome P450 monooxygenases enable the regio- and stereo-selective hydroxylation of C-H bonds, they are of outstanding interest for the synthesis of pharmaceuticals and fine chemicals. Epoxidation of BPA catalyzed by P450.

coli BL21(DE3) .