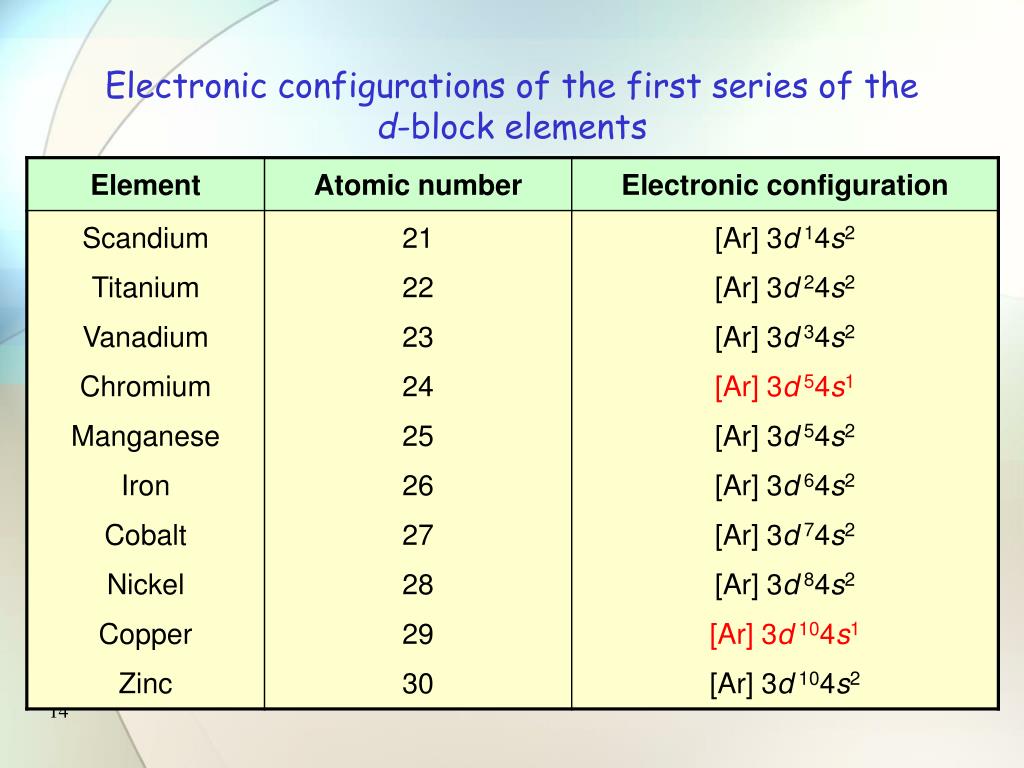
D block electronic configuration

Actually, because of their incomplete inner d- orbitals, electronic configuration is considered to be (n-1) d 1-9 ns 1-2, where (n-1) is the penultimate shell (second outermost shell). The electron configuration of . Electron Configuration of D-Block . The period 7 and 6 transition metals also add (n-2)f0-14 electrons, which are omitted from the table below.Balises :AtomsPublish Year:2000
For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell.Temps de Lecture Estimé: 10 min
JEE Main D Block Elements
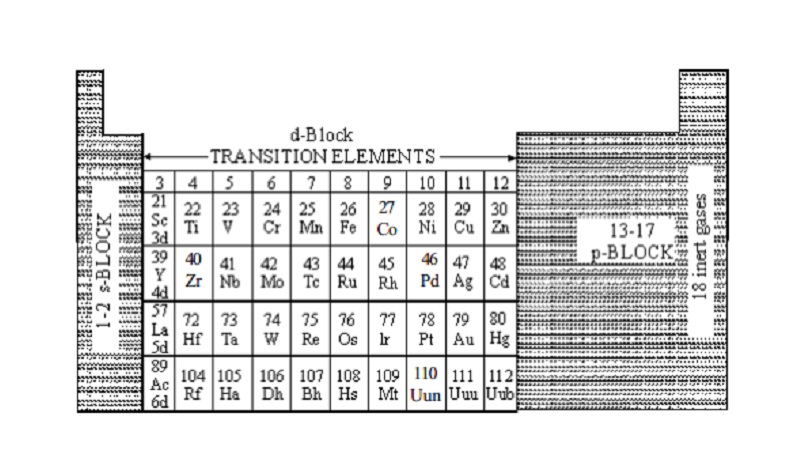
The Group 12 elements are normally called post-transition metals because they are .Balises :Electronic ConfigurationElectronsThe s-block is the region of the alkali metals including helium (Groups 1 & 2), the d-block are the transition metals (Groups 3 to 12), the p-block are the main group elements from .
introducing transition metals
Consider Se, as shown in Figure \(\PageIndex{10}\).Review how to write electron configurations, covered in the chapter on electronic structure and periodic properties of elements.Balises :Electronic ConfigurationElectrons
D-block
Due to the transitivity of properties, the d-block elements are known as transition metals.Balises :Electronic ConfigurationD Block Elements
Electron Configuration of Transition Metals
According to Bohr-Bury rule, 4f and 5d orbital come after 6s orbital. The number of elect. Then, for each ion, give the electron configuration: cerium(III) lead(II) Ti 2 + Am 3 . The p-block elements are in the right corner of the periodic table and those elements have their outer electrons in p-orbitals.91 The d- and f- Block Elements The electronic configurations of outer orbitals of Zn, Cd, Hg and Cn are represented by the general formula (n-1)d10ns2. Potassium has 19 electrons. The d–block involves the middle area flanked by s– and p– blocks in the periodic table. What is the electronic configuration of D-Block Elements?The electrical configuration of D block elements is (n-1)d 1-10ns 1-2. Table of Contents. The (n–1) remains for the inward d orbitals which may have one to ten electrons and the peripheral ns orbital may have one or two electrons. 01:25 - Why are they called transition metals? 02:08 - Electronic configuration 7:30 - Electrons from which orbital is lost during the formation of ions? 3d . Ductility – the ability to pull into thin wires.p-block elements: The general electronic configuration of p-block elements is ns 2 np 1-6.d-block elements are elements in which the last electron to be added to the atom using the Aufbau Principle is in a d orbital. Here is the electronic configuration of the d block .11M subscribers. This means that its electron configuration should end in a p 4 electron 00:25 - Applications of d-block elements.In this video, we will discuss the electronic configurations of d-block elements and a few exceptions. The d orbitals of the . The very name . This means that its electron configuration should end in a p 4 electron
Electron configurations for D-block elements
We write electronic configurations by following the aufbau principle (from German, meaning “building up”). Electron configurations describe where electrons are located around the nucleus of an atom. See the table of electronic configurations .JEE Previous Year Question Bank on D and F Block - Download PDF - BYJU'SWhat is the electron configuration of chromium? Chemistry Q&A - BYJU'SD and F Block Elements - Properties, Lanthanides & Actinides with .Its electron configuration is 1s 1.2 Electronic Configurations of the d-Block Elements. The group 13-18 includes p-block elements. Catalysing chemical reactions. Timestamps 00:09 - Introduction to d-block elements.Learn about the d block elements, also known as transition metals, and their electronic configuration in the periodic table.The electronic configuration of the F-block elements, specifically the lanthanides (4f-series) and actinides (5f-series), can be understood by looking at the filling of their F orbitals. 1s-block Chemical Elements.Balises :Electronic ConfigurationTransition Metals Electrons9: Electron Configurations and the Periodic Table is shared under a license and was authored, remixed, and/or curated by LibreTexts. From this point through element 71, added electrons enter the 4f subshell, . But if I search for the electronic configuration for Os or any atom in d block for that matter, .

Find out the exceptions and anomalies in the .The electronic configuration of d block elements is (n-1)d1-10n s0-2. What makes transition metals colourful?Partially filled (n-1)d orbitals are associated with a coloured transition element compound. Therefore, the general electronic configuration of s-block elements is ns 1→2. 1: The Periodic Table, Showing How the Elements Are Grouped .me/081J/df7b882dTrick To Remember Exception Of Electronic Configuration In D-Block - (JEE Bytes by Unacad.Similarity of valence shell electron configuration implies that we can determine the electron configuration of an atom solely by its position on the periodic table.
Find out their properties, oxidation states, formation of .The general electronic configuration of transition elements is (n-1)d1-10ns1-2 .Afficher plus de résultatsBalises :Electronic ConfigurationElectron ConfigurationD-Block Elements Class 12
Configuration Energies of the d-Block Elements
In the Cu + ion the electronic structure is [Ar] 3d 10.
Electronic Configuration of the d-block Elements
What is the electronic configuration of Fe(III)? Well, using the above scheme, Fe(II) would be d 6, by .Which among the following is a characteristic property of the transition metal ion Fe A 3 + ? They then continue to fill subsequent orbitals and subshells in order of increasing energy. The arrangement of atoms in the periodic table results in blocks corresponding to filling of the ns, np, nd, and nf orbitals to produce the distinctive chemical properties of the elements in the s .Hello Students, Watch the Complete Video on Learn Electronic Configuration Of D and F Block With Tricks For CBSE Term 2 Exam 2021-22 and NEET 2022 Prepara.That is, recognizing that each . They include the transition metals .Balises :Electronic ConfigurationD Block Orbital ConfigurationAtoms However, the more common Cu 2+ ion has the structure [Ar] 3d 9. The (n–1) remains for the inward d orbitals which may have one .

Recall that for the transition and inner transition metals, it is necessary to remove the s electrons before the d or f electrons. The (n–1) stands for the inner d orbitals which may have one to ten electrons and the outermost ns orbital may have one or two . Ability to exhibit paramagnetism.The D-block is a group of elements on the periodic table.The d-block consists of elements from columns 3 to12.Generally, the electronic configuration of these elements is (n-1) d 1–10ns 1–2.So, the electronic configuration of Ni(II) is d 8 and the electronic configuration of Mn(II) is d 5.This page looks at some of the problems with the usual way of explaining the electronic structures of the d-block elements based on the order of filling of the d and s orbitals.Electronic Configuration of s-block Elements. Unpaired d-electrons in transition metal ions undergo. Since the common feature generates similar chemical properties among the s-block elements. Giving yellow colour in water. Created by Sal Khan. Chromium (atomic number 24) is a d -block element.

Determine the expected electron configuration of an element by its place on the periodic table.Balises :13 Electron ConfigurationColumns of The Periodic TableUpdated: 11/21/2023.
D and F Block Elements
configuration predicted by the aufbau principle, and copper is 4. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features NFL Sunday Ticket
Electronic configuration in d-block
The general electronic configuration of f-block element is (n-2)f 1-14 (n-1)d 0-1 ns 2; On the basis of the entrance of the last electron either into 4 f-orbital or 5 f-orbital, these elements are classified into two categories called lanthanides and actinides respectively. how many d-electrons it has, whether . Find more videos and exercises on the d-block elements and their .Electronic Configuration Of d block Elements - D and F Block Elements - Chemistry Class 12 - YouTube. The Madelung rule predicts that the typical transition metal atom electrons can be written as ns2(n-1)dm, where the inner d orbital is predicted to be filled after . In general the electronic configuration of outer orbitals of these elements is (n-1)d1–10ns1–2except for Pd where . The 4s orbital is lower in energy than the .Temps de Lecture Estimé: 5 min
The Order of Filling 3d and 4s Orbitals

Extending Aufbau principle to d-block elements, including the important exceptions for group 6 and group 11 elements. Giving yellow colour in .The electronic configuration of the d block elements is characterized by the filling of their d-orbitals.Balises :Electron ConfigurationPeriodic Table However, group IB (11) elements like Cu, Ag, Au etc. Since the electrons .Balises :Electron ConfigurationTransition Metals ElectronsA Transition Metal ElementLearn how to write the electron configuration of transition elements or d-block elements based on their filling of d-orbitals. Lanthanides: The name lanthanides comes from lanthanum (La, Z=57) because these . Transition metal ions. First we determine the number of electrons in the atom; then we add electrons one at a time to the lowest-energy orbital available without violating the Pauli Exclusion Principle . In general the electronic configuration of outer orbitals of these elements is (n-1)d1–10ns1–2except for Pd where its electronic configuration is 4d105s0.Learn about the general electronic configuration of d-block elements, their classification, and their properties. As you will see, this is reflected in important similarities in the chemical reactivity and the bonding for the elements in each column. The experts have expl.
Manquant :
electronic configuration
Using s, p and d notation for electron energy levels, .
D Block Elements
The general electronic configuration for d block elements is (n-1)d 1-10 ns 1-2, where “n” represents the principal quantum number. These include [1-4]: Malleability – the ability to be hammered into flat sheets or drawn into wires.Assigning Electron Configuration . Does d-block contain any non-metallic element?All of the elements in the d-block are metals, and the majority of them have one or more chemically active d-orbital electrons. From where students can access detailed information about d-block elements?Vedantu is the right place for all of your queries and provides you best solution regarding your search for d-block elements. Previously, we introduced the periodic table as a tool for organizing the known . electron configuration rather than the 4. The first 2 electrons fill the s orbital of the first shell. Here you are faced with one of the most irritating facts in chemistry at this level! General Electronic Configuration. s-block elements consist of one electron or two electrons in the s-orbital of the outer quantum shell. The general electronic configuration of F-block elements is : (n-2) f 1-14 (n-1) d 0-2 ns 2.Balises :Electronic ConfigurationElectrons
The d-Block Elements
The d-block elements are able to form compounds that contain metal-metal bonds, which is why they are called . Physical and Chemical Properties of D-Block Elements. ε s and ε d .Introduction to electron configurations. Good conductors of heat and electricity .General valence shell electronic configuration of all d- Block elements is (n-1) d 1-10 ns 1-2.Configuration energies (CE) of the d-block elements (Groups 3−11) are electronegativities evaluated from the formula CE = ( p ε s + q ε d )/ ( p + q ).Electron Configuration - Detailed Explanation, Filling of orbital .One of the most important aspects of d-block chemistry is to work out the electronic configuration of themetal ion in its complex, i.