Define in vitro diagnostic device

The most significant changes in the regulation include: 1.-In-vitro Diagnostic Medical Devices Directive 98/79/EC - Commission Decision of 07/05/02 on Common Technical Specifications (CTS) for IVD Medical Devices The former is transposed into Irish law by way of: - S.
MHRA also oversees UK approved bodies.
Guidance on the regulation of IVD medical devices in GB
In September 2012, the European Commission (EC) published a proposal for a new regulation on in vitro diagnostic . At the same time, this Regulation sets high standards of quality and .This document was prepared by Technical Committee ISO/TC 212, Clinical laboratory testing and in vitro diagnostic test systems, in collaboration with the European .The Investigational Device Exemptions (IDE) regulation, Title 21, Code of Federal Regulations (21 CFR) Part 812, sets forth regulatory requirements for studies of .An IVD medical device is defined in the IVDR as “any medical device which is a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, piece of .

LFIAs represent a well-established and very appropriate technology when applied to a wide variety of in vitro diagnostics (IVD) or field-use applications. In this process, the same actions must also be taken for IVD devices as for other Medical Devices, namely: Identify an Issuing Entity that . Read the meeting statement from 7 December 2023 meeting of the Advisory Committee on Medical Devices (ACMD).A clinical study that trials a device in or on a person, is called a clinical investigation.Per Section 201(h)(1) of the Food, Drug, and Cosmetic Act, a device is: An instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article .In vitro diagnostic medical devices (IVDs) are tests used on biological samples to determine the status of a person's health.
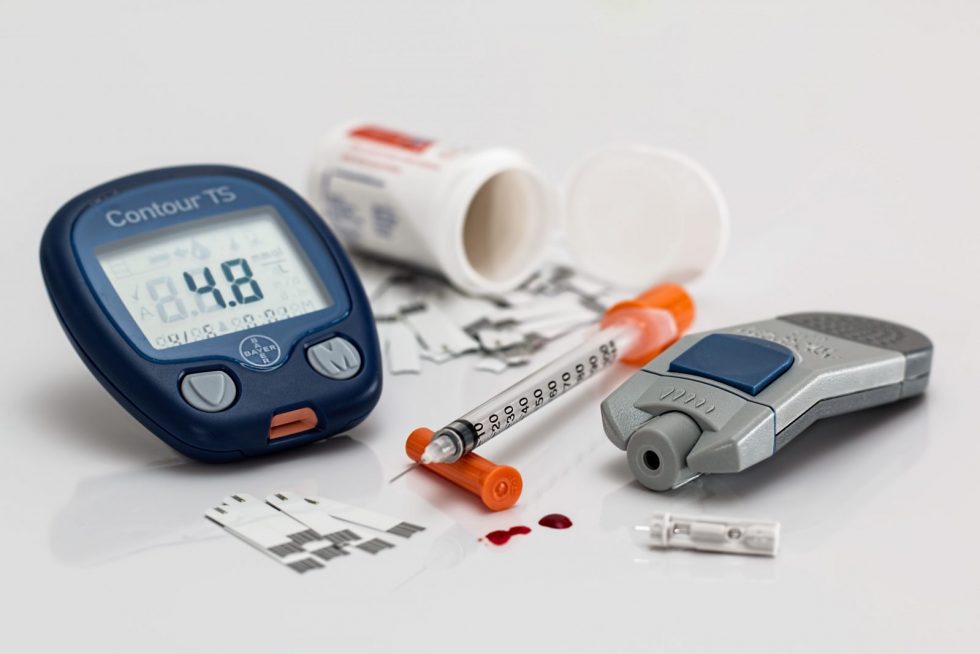
An important category of medical devices, distinct from the therapeutic products described and discussed in other chapters, are the in vitro Diagnostic Devices (IVDs). A study that validates an in vitro diagnostic for medical use is a . Published date.
Manquant :
defineIn vitro diagnostics (IVDs) are tests that can detect disease, conditions and infections.Medical Devices
Meeting statements. The In Vitro Diagnostic Regulation (IVDR) replaced the IVDD and entered into force on 26 May 2017 with 26 May 2022 as date of application.Regulation (EU) .those reagents, instruments and systems intended for use in the diagnosis of disease, or other .
in vitro diagnostic device Definition
Introduction to In Vitro Diagnostic Devices
The industry employs about 75 000 people in Europe, and generates some €11 billion in revenue per year.Manufacturers of in vitro diagnostic (IVD) medical devices supply users with information to enable the safe use and expected performance of their devices.From May 26, 2023, the UDI is mandatory for class D IVD devices, while from May 26, 2025 for class B and C IVDs. IMDRF/RPS WG/N13.These Regulations make provision for the implementation in respect of Northern Ireland of Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission Decision 2010/227/EU (“Regulation (EU) 2017/746”).1007/978-3-319-19737-1_1
In Vitro Diagnostic Medical Devices Regulation
Placing your In Vitro Diagnostic Medical Device on the market As an IVD manufacturer, you must ensure that your product meets the relevant regulatory requirements before being placed to the market.
MD vs IVD
Introduction to In Vitro Diagnostic Devices
This guidance, relating to the application of Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR) addresses the classification of in vitro diagnostic . Transitional provisions. Class C covers different of high-risk IVD devices which present a lesser risk to the wider population. Manufacture is relatively easy: Equipment and processes are already developed and available.
In vitro diagnostics
In Vitro Diagnostics
device on the market In Vitro Diagnostic Medical Device Examples: • pregnancy tests • blood glucose monitors Definition*: ‘In vitro diagnostic medical device’ means any . The advantages of the .In Vitro Diagnostic (IVD) Device Studies - Frequently Asked Questions.0 Definitions Accessory for an IVD Medical Device: accessory for an in vitro diagnostic medical device’ means an article which, whilst not being itself an in vitro diagnostic . NOTE 2: Includes both vacuum and non-vacuum primary sample collection devices.Covers the latest EU regulatory requirements and standards for developing medical devices; Includes information on patents and licenses; Covers regulations for medical .
Manquant :
defineHow to Determine if Your Product is a Medical Device
Accessories on their own will not provide diagnostic information and it is this that will .
Manquant :
defineIn vitro diagnostic medical devices
The European Union In Vitro Diagnostics Regulation
Definition: In vitro diagnostic products are those reagents, instruments, and systems intended for use in diagnosis of disease.ISO 18113-1:2009 In vitro diagnostic medical devices - Information supplied by the manufacturer (labelling) – Part 1: Terms, definitions and general requirements. In vitro diagnostics can detect . Product scope .
Manquant :
defineDiagnostic in vitro — Wikipédia
Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 on in vitro diagnostic medical devices and repealing . IMDRF code: IMDRF/RPS WG/N13FINAL:2019 (Edition 3) Published date: 21 March 2019.(2) This Regulation aims to ensure the smooth functioning of the internal market as regards in vitro diagnostic medical devices, taking as a base a high level of protection of health for patients and users, and taking into account the small and medium-sized enterprises that are active in this sector.National Medicinal Regulatory Agencies (MRA) are responsible for evaluation, control and approval of in vitro diagnostic devices (IVD).In Vitro Diagnostic Medical Device Market Authorization Table of Contents (IVD MA ToC) IMDRF Code.

‘in vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, piece of equipment, software or system, whether used alone or in combination, intended by the manufacturer to be used in vitro for the examination of specimens, including blood and .The new EU Medical Device Regulations (MDR)[] and in vitro Diagnostic Regulation (IVDR),[] 2017 make notified bodies, competent authorities and the European Commission more responsible than ever before for the safety of medical devices, including in vitro Diagnostics.
In Vitro Companion Diagnostic Devices
The European Commission has adopted 2 new Regulations – the Medical Devices Regulation (MDR) and the In Vitro Diagnostic Medical Devices Regulation (IVDR) - to . 304 of 2001, European Communities (In-vitro Diagnostic Medical Devices) Regulations, 2001 The latter is a Commission . All publications. Article 5(4) of the Windsor .In Vitro Diagnostic Regulation. in vitro diagnostic device or (“IVDD”) is defined as reagents, instruments, and systems intended for use to measure [***] in serum or plasma for use in the diagnosis of disease or other conditions, including a determination of the state of health, in order to cure, mitigate, treat, or prevent disease or its sequelae. This guidance is intended to assist (1) sponsors who are planning to develop a therapeutic product1 (either a novel product or an existing product with a new .Companion diagnostics can: monitor response to treatment with a particular therapeutic product for the purpose of adjusting treatment to achieve improved safety or effectiveness. The IVDR “brings EU legislation into line with technical advances . The Regulation applies from 26 May 2022, following a five-year transition period.What are in vitro diagnostic medical devices? In vitro diagnostic medical devices (IVD) are tests used on biological samples (such as tissues, blood or urine) to determine the .In vitro diagnostic medical devices are tests used on biological samples to determine the status of a person's health.The In Vitro Medical Devices Regulation (EU) 2017/746 (IVDR) is a new regulation that will create a robust, transparent, and sustainable regulatory framework that “improves clinical safety and creates fair market access for manufacturers and healthcare professionals” (1).In Vitro Companion Diagnostic Devices. To help with a smooth transition from the previous to the . In Vitro Diagnostic Medical Device Market Authorization Table .Le diagnostic in vitro (en anglais, in-vitro diagnostic ou IVD) regroupe toutes les techniques, tous les appareils ou les dispositifs utilisés sur des échantillons de .‘Accessory’ means ‘an article which, whilst not being an in vitro diagnostic medical device, is intended specifically by its manufacturer to be used together with a device to enable that device to be used in accordance with its intended purpose.
In vitro diagnostic medical devices
Medical devices
Publication of MDCG 2022-12 Guidance on harmonised administrative practices and alternative technical solutions until Eudamed is fully functional (for Regulation (EU) . IVDs are, by definition of the Food and Drug Administration (FDA), “. Before it can issue a CE .Specimen Receptacle: apparatus specifically intended by а manufacturer to obtain, contain and preserve a body fluid or tissue for in vitro diagnostic examination NOTE 1: Includes devices intended to store a primary sample prior to examination. The IVDR differs in several important ways from the EU’s previous directive on in vitro diagnostic medical devices.Class D In-Vitro diagnostic is related to life-threatening conditions and, in particular transmissible agents in blood and other biological materials intended to be re-inserted to administrated in the body.The In Vitro Diagnostic Devices Regulation (Regulation (EU) 2017/746) introduces a new classification system for companion diagnostics and the obligation to undergo a conformity assessment by a notified body.










