Definition of nonpolar covalent compound kid version
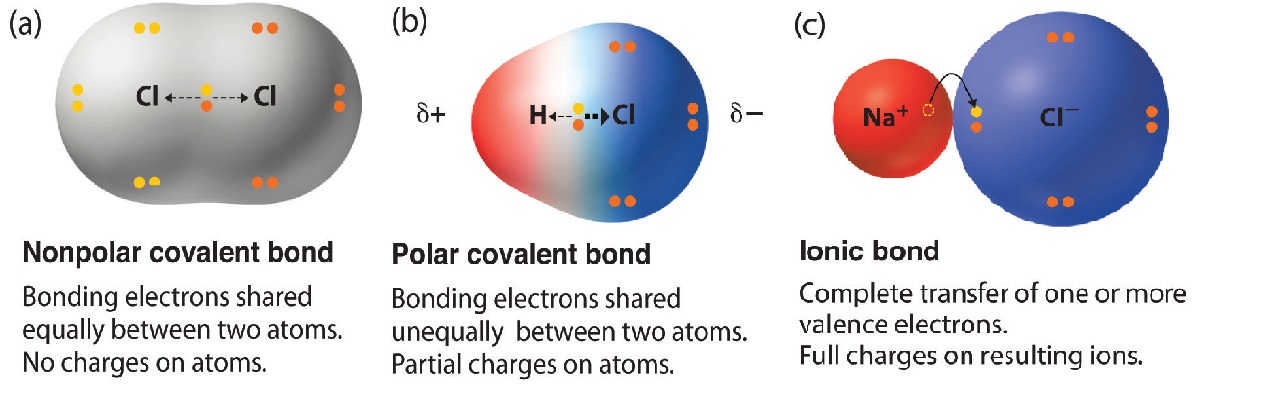
Because of these features of nonpolar . Some covalent compounds might dissolve in water due to the formation of intermolecular hydrogen bonding with water molecules.The bottom three molecules are nonpolar. Thus, in an atom, the number of electrons shared . The two chlorine atoms share the pair of . noun, plural: nonpolar compounds. A full outer shell .Compounds that contain covalent bonds exhibit different physical properties than ionic compounds. (b) Symbols δ+ and δ– indicate the polarity of the H–Cl bond. The elements with the highest ionization energies are . Physical state: These compounds can exist as solids due to greater force of interactions. The term ‘polar compound’ can be defined as a chemical species which consists of two or more atoms that are held together by covalent bonds that are polar in nature due to the unequal sharing of electrons. Another non polar molecule shown below is boron trifluoride, BF 3. Covalent compounds do not dissolve in polar solvents but can dissolve in non-polar solvents. dinitrogen pentoxide., H 2 gas), but chemists consider any bond between atoms with a .
Also Read: Covalent Bonds Properties of Polar Covalent Compounds. These compounds are called non . Join / Login >> Class 11 >> Chemistry >> Chemical Bonding and Molecular Structure >> Covalent Bond >> Polar and Non Polar .The bond length is determined by the distance at which the lowest potential energy is achieved.
Different covalent compounds exhibit isomerism due to the directional nature of the bonds.Read formulas, definitions, laws from Covalent Bond here. Solve Study Textbooks Guides. In a nonpolar covalent bond, the distribution of . 3 In the polar covalent bond of HF HF, the electron .Non-polar covalent bonds, with equal sharing of the bond electrons, arise when the electro-negativities of the two atoms are equal. Covalent Compounds. This is a nonpolar covalent bond. Melting and boiling points: These have greater melting and boiling point than non-polar compounds.A nonpolar covalent bond is one in which the electrons are shared equally between two atoms. The number of bonds an element forms in a covalent compound is determined by the number of electrons it needs to reach octet. Click here to learn the concepts of Polar and Non Polar Covalent Compounds - On the Basis of Electronegativity from Chemistry. Polar molecules contain polar covalent or ionic bonds that are arranges so their partial charges do not cancel each other out.Whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity.Between two identical nonmetal atoms or between different atoms a nonpolar covalent bond can occur. A covalent bond is formed between two atoms by sharing electrons.Many covalent compounds are liquids or gases at room temperature and as solids they are usually softer than ionic solids. Both of these molecules would be oil or fat-soluble.1) have similar electronegativities.12: Acids and Bases - The Lewis Definition. Ionic compounds are good conductors of electricity when dissolved in water but most covalent compounds are poor conductors of electricity in any state since the molecules do not have an overall charge. Not ionizing when dissolved in water. Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom . When it is large, the bond is polar covalent or ionic. The small, black dots indicate the location of the hydrogen and chlorine nuclei in the molecule. For example, both hydrogen and oxygen are nonmetals, and when they combine to make water, they do so by forming . This definition covers Brønsted-Lowry proton transfer reactions, but also includes reactions in which no proton transfer is involved. (b) The molecular structure is square planar with the lone pairs directly across from one another.1 (ionic), respectively. Mathematically, dipole moments are vectors; they possess both a magnitude and a direction. The electron density is greater around the chlorine nucleus.Carbon–hydrogen bonds, for example, are relatively nonpolar because carbon (EN = 2. Bonds between carbon and more electronegative elements such as oxygen (EN = 3. When two atoms are bound together via a covalent . If this relative attraction is great enough, then the bond is an ionic bond.Pure covalent bonds (nonpolar covalent bonds) share electron pairs equally between atoms.Temps de Lecture Estimé: 5 min
Bond Polarity ( Read )
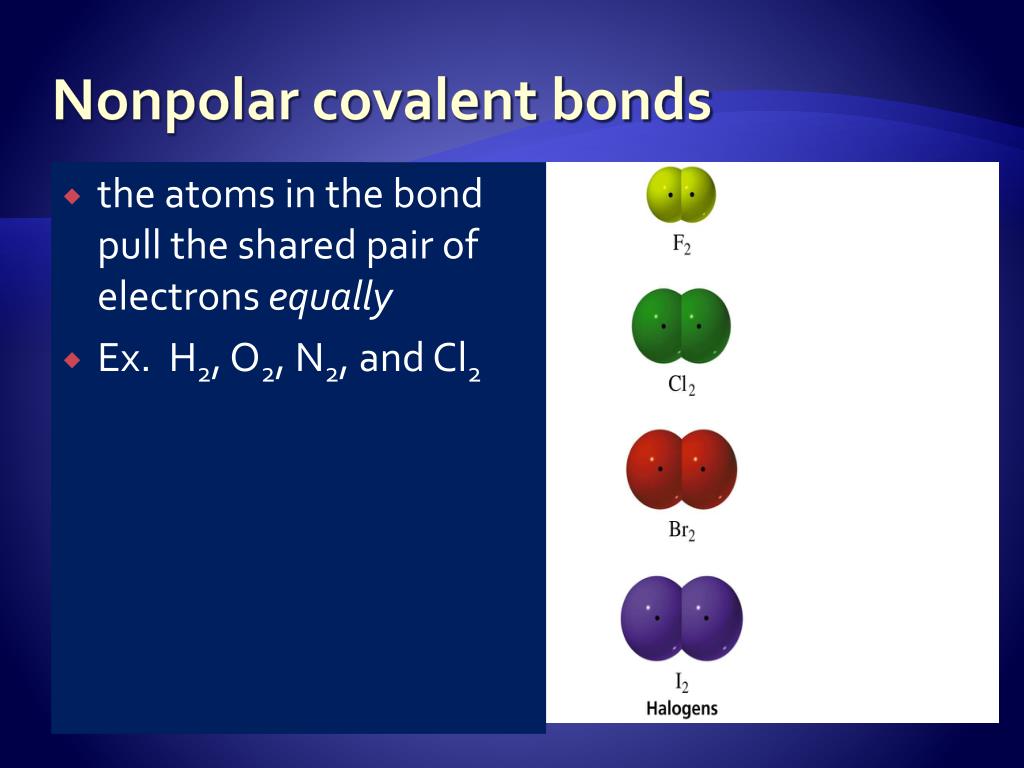
These compounds are called non-polar covalent compounds.
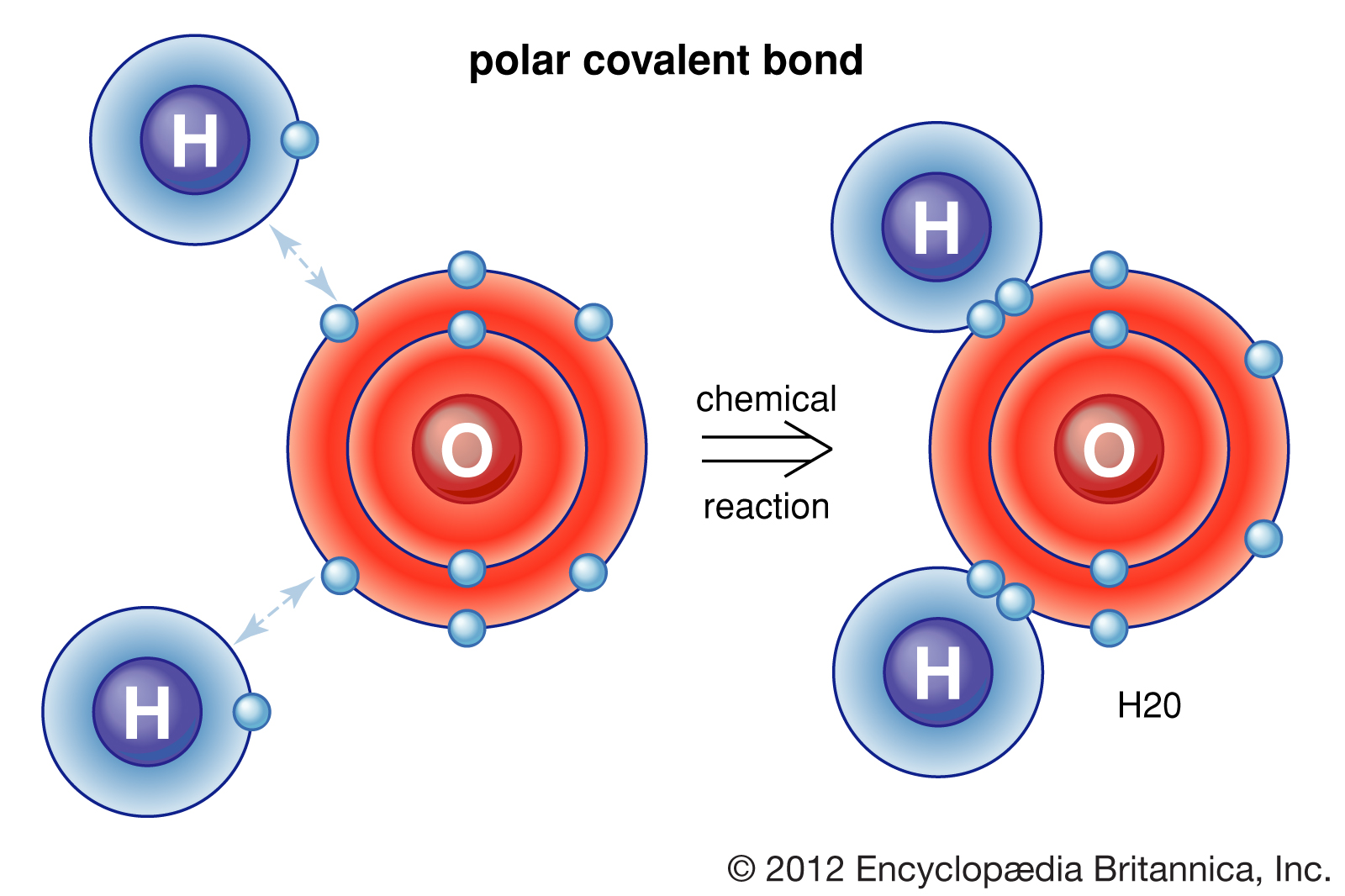
This partial ionic character of covalent bonds increases with the difference in the electronegativities of the two atoms.
Covalent Bond- Definition, Properties, Types, Examples
The dipole moment is defined . These are symmetrical molecules that have dipoles that cancel.5) and hydrogen (EN = 2.Define ionic and molecular (covalent) compounds; Predict the type of compound formed from elements based on their location within the periodic table; Determine formulas for .Polar bonds are the dividing line between pure covalent bonding and pure ionic bonding. So long as the .A nonpolar covalent bond is a covalent bond in which the bonding electrons are shared equally between the two atoms. An example is H 2 gas, where H − H both have same electro-negativities.Nonpolar Covalent Bond: A bond is deemed nonpolar if the difference in electronegativity is extremely minor, less than 0. Conductivity: They conduct electricity in the solution state due to the mobility of ions. A pair of electrons that is shared between two atoms is . They will also not convert into ion s in a solution. The two atoms in a nonpolar . Write the molecular formula for each compound. Nonpolar molecules may contain any type of chemical bonds, but the partial . A polar covalent bond is one in which one atom has a greater attraction for the electrons than the other atom. 1 Polar versus Nonpolar Covalent Bonds. (a) The electrons in the covalent bond are equally shared by both hydrogen atoms.A non-polar covalent bond is a type of chemical bond that is formed when electrons are shared equally between two atoms. H forms only one bond because it needs only two electrons. 4: A nonpolar covalent bond is one in which the distribution of electron density between the two atoms is equal. This occurs when the two atoms have similar (or the same) electronegativity values.The five atoms are all in the same plane and have a square planar molecular structure. BF 3 is a trigonal planar molecule and all three peripheral atoms are the same.
Non-polar covalent compounds Non-polar covalent bonds, with equal sharing of the bond electrons, arise when the electro-negativities of the two atoms are equal. In chemistry, a polar bond is a type of covalent bond between two or more dissimilar atoms, in which electrons are shared unequally. Electronegativity. Nonpolar molecules may contain any type of chemical bonds, but the partial charges cancel each other out. (a) XeF 4 adopts an octahedral arrangement with two lone pairs (red lines) and four bonds in the electron-pair geometry.
Non-polar Covalent Bond
Non-polar covalent compounds are . A bond between two atoms or more atoms is non-polar if the .Nonpolar Covalent Bonds.
Covalent Bonds
(b) The chlorine atom attracts the electrons in the bond more than the hydrogen atom does, leading to an imbalance in the electron distribution. 3: Some examples of polar and nonpolar molecules based on molecular geometry. An example is water, where hydrogen (H) and oxygen (O) bond together to make (H 2 O). A compound comprised of molecule s linked through chemical bond s arranged in such a way that the distribution of charges are symmetrical.Temps de Lecture Estimé: 3 min As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between atoms, within a covalent bond: A nonpolar covalent bond (Figure \(\PageIndex{1a}\))is one in which the electrons are shared equally between two atoms.The asymmetrical charge distribution in a polar substance such as HCl produces a dipole moment Qr Q r is abbreviated by the Greek letter mu (µ). The absolute values of the electronegativity differences between the atoms in the bonds H–H, H–Cl, and Na–Cl are 0 (nonpolar), 0.Kids Encyclopedia Facts.When the difference is very small or zero, the bond is covalent and nonpolar. Another way the octet rule can be satisfied is by the sharing of electrons between atoms to form covalent bonds.
Covalent bond Facts for Kids
phosphorus pentachloride.Polar compounds are chemical compounds that are held together by polar covalent bonds.A covalent bond that has an equal sharing of electrons (part (a) of Figure \(\PageIndex{1}\)) is called a nonpolar covalent bond. American Heritage® Dictionary of the English. chlorine trifluoride.
Nonpolar compound
If there is no . For each molecule, there are different names for pairs of electrons, depending if it is shared or not. In a polar covalent bond, sometimes simply called a polar bond, the distribution of electrons around the molecule is no longer symmetrical. Because the attraction between molecules, which are electrically neutral, is weaker than that between electrically charged ions, covalent compounds generally have much lower melting and boiling points than ionic compounds.Nonpolar covalent bonds occur when two atoms share electrons evenly.

5) and nitrogen (EN = 3.Non-polar bonds are also a type of covalent bond. Unlike polar bonds, non-polar bonds share electrons equally.; A polar covalent bond (Figure \(\PageIndex{1b}\)) is one in which one atom has a . 2: (a) The distribution of electron density in the HCl molecule is uneven.A nonpolar covalent bond is one in which the distribution of electron density between the two atoms is equal. In fact, many covalent .

1 Some examples of nonpolar molecules based on molecular geometry (BF 3 and CCl 4 ). If the individual bond dipole moments cancel one another, there is no net dipole moment. A broader definition is provided by the Lewis theory of acids and bases, in which a Lewis acid is an electron-pair acceptor and a Lewis base is an electron-pair donor. The degree to which .
Polar bond Facts for Kids
1: The potential energy of two separate hydrogen atoms (right) decreases as they approach each other, and the single electrons on each atom are shared to form a covalent bond., one atom is slightly negatively charged and the other is slightly positively charged.Nonpolar covalent bonds form between two atoms of the same element or between different elements that share electrons equally.9 (polar covalent), and 2.
Polar and Nonpolar Molecules
Electronegativity is a measure of the tendency of an atom .Polar bonds form between atoms of elements with different electronegativity values. A compound comprised of molecule s linked through chemical bond s arranged in such a way that the distribution .0), by contrast, are polarized so that the bonding electrons are drawn away from carbon toward the . nonpolar compound synonyms, nonpolar compound pronunciation, nonpolar compound translation, English dictionary definition of nonpolar compound., electrically uniform—while those between unlike atoms are polar—i. A covalent bond is a chemical bond between two non-metal atoms. Not characterized by a dipole: a nonpolar covalent bond. Technically, nonpolar bonding only occurs when the atoms are identical to each other (e. Contributors and Attributions.