Different atomic models
When the exact structure of the atom remained quite unknown, models were proposed based on experimental evidence of the properties of matter. In this segment, the students learn about different models of the atom, including Dalton’s model, Thomson’s model, Rutherford’s . It was put forward by the English physicist and chemist John Dalton. 412K subscribers.What Are The Different Atomic Models?
Models of the atom
The structure of the periodic table, which was determined empirically, can be derived theoretically from first principles. Table salt is a combination of two separate elements with unique physical and chemical properties.Thomson’s model was the first to suggest that atoms were not indivisible and had a substructure. Schroedinger 9. Discovery of the electron and nucleus. Planetary model. It contains lots of video media. The darker the region, the more likely electrons are to be found .
The atom is mostly empty space.Temps de Lecture Estimé: 10 min
Modèles atomiques, chronologie des modèles de l'atome
The electrons are free to orbit the nucleus in a manner .3 Rutherford’s model of the atom.
History of atomic theory
Atomic Model
Rutherford conducted an experiment in which he utilized alpha particles to scatter off of a sheet of gold. However, they should be able to explain the basic properties of matter. Surprisingly, while most of the alpha particles were indeed undeflected, a very small . The current theoretical model of the atom involves a dense nucleus surrounded by a probabilistic . Some alpha particles were . Discovery of sub-atomic particles. The page below is a brief overview on the history of atomic theory.This quantized atomic model, also known as the planetary model of the atom, was used to explain why the electrons' orbits are stable and why elements absorb and .Earnest Rutherford Circa ~ 1911. Ernest Rutherford model- Nuclear model of an atom. Such models would be purely theoretical constructs. They are: =>John Dalton's atomic model: Dalton´s Billiard Ball (Solid Sphere) Model =>J.Nuclear model (1911) Planetary model (1913) Quantum mechanical model (1926-present) Atomic model: John Dalton. Furman University. Scientists needed a fundamental change in their way of thinking about the electronic structure of atoms to advance beyond the Bohr model. Electrons can only exist in certain discrete energy states.Wolfgang Pauli (1900–1958) Austria — exclusion principle.
Five Types of Atomic Models
Ans: The five atom is models are as follows: John Dalton’s atomic model.

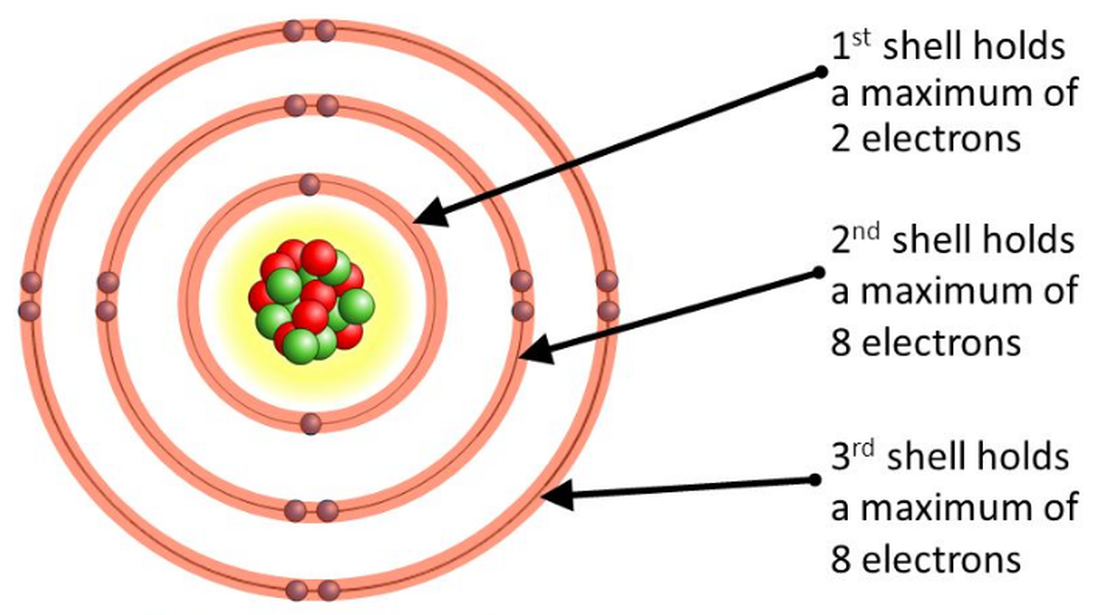
The ground states of all elements follow the pattern of the excited states in the hydrogen atom.The atomic model consists of a nucleus containing protons and neutrons, surrounded by electrons in shells.Understand Chadwick’s atomic model in relation to protons, neutrons and electrons and incorporate it by calculating relative mass and charge. The second, chlorine, is a toxic . Democritos (Philosopher) It . In the very beginning of the 1800s, John Dalton proposed that atoms were like tiny, hard billiard balls. Maquette de Démocrite d'Abdère (an 450 av.The results of these measurements indicated that these particles were much lighter than atoms (Figure 2. As we'll discuss later in the article, atomic electrons exist at specific energy levels. Notes about this page. (Setup: 5min, Activity: 10 min, Cleanup: 5 min) Materials: $8. In this mission, you’ll look at how atoms are put together inside a material, and how that affects its properties. Find out how each . Table of contents. Structure of the Atom. • Average Atomic Diameter ~ 10-8 cm.1: Historical Development of Atomic Theory.Most of the alpha particles went nearly straight through with relatively little deflection. You’ll do this by creating a model made of candy, which will show some of the same patterns a scientist would see with a (non .Ci-dessous, nous présentons la chronologie des modèles atomiques les plus importants : 1.67 Jeff Wiener* CERN, SWITZERLAND Received: July 9, 2020 Revised: November 11, 2020 Accepted: December 13, 2020 Abstract: This article presents an international study that documented the conceptions of atomic models held by 1062 in-service high school . External links.5 Different Atomic Models- Theories, Diagram & Structure of Atom.Rutherford’s Atomic Model. An example of such a compound is table salt. According to this theory, all substances are made up of atoms, which are indivisible and indestructible. Different Atomic Models and Their Explanation.
5: Models of the Atom
Rutherford model, description of the structure of atoms proposed (1911) by the New Zealand-born physicist Ernest Rutherford.J Thomson’s atomic model- Plum pudding model. The first postulate states that all matter is composed of atoms.4: Thomson Model of the Atom; 5. The Atomic Model. Erwin Schrödinger’s model-Quantum model. Thomson's model: Plum Pudding model =>Ernest Rutherford's model: Nuclear model =>Niels Bohr's model: Planetary model =>Erwin Schrödinger's .Though several alternative models were advanced in the 1900s by Kelvin . Though our graphic starts in the 1800s, the idea of atoms was .
Segment A: Atomic Models
comRecommandé pour vous en fonction de ce qui est populaire • Avis Matter is made of small indivisible atoms. Elizabeth Gordon. Further reading. Dalton’s Atomic . Different atomic species. The model described the atom as a tiny, .In 1808, John Dalton (1766 – 1844) proposed his atomic theory, which served as an explanation for these phenomena.

Histoire d’un modèle, le modèle de l’atome | Lelivrescolaire.Thomson’s plum pudding atomic model.1 Atomic models.
The atomic model
3: Dalton Model of the Atom; 5. (c) In the cathode ray, the beam (shown in yellow) comes from the cathode and is accelerated past the anode toward a fluorescent scale at the end of the tube.5: Rutherford Model of the Atom; 5.6: Atomic Notation; .1 Modern Atomic Theory. Quantum Mechanics.4 Bohr’s model of the atom.Unit 3: Atomic Structure. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. An atomic mass unit (amu amu) is defined as one-twelfth of the mass of a carbon-12 atom. 1 gives the properties and locations of electrons, protons, and neutrons.inside the nucleus.2: Quantum Mechanical Model of the Atom; 5.

frlelivrescolaire.
The History of the Atom
Each thin band in . His view of completely solid atoms seems like a very basic idea now, but in 1803 it was groundbreaking.2 Plum pudding model. The Bohr model represents the particle nature of electrons. Compare different theories regarding electron . It stated that matter is made up of small indivisible particles called ‘atoms’.The five main atomic models are: Dalton's atomic model .However, the atomic nucleus had not been discovered yet and so the “plum pudding model” was put forward in 1904. Figure \(\PageIndex{5}\): The atomic emission spectra for various elements. Learning Objectives. This unit also includes an overview of protons, neutrons, and electrons, as well as a periodic table of elements. (c) In the cathode ray, the beam (shown in yellow) comes from the . It is named plum pudding model (Fig.In fact, Bohr’s model worked only for species that contained just one electron: H, He +, Li 2 +, and so forth.Learn about the history and development of atomic models, from Rutherford's nuclear atom to Bohr's quantized atom. The discovery of the electron led Thomson to the development of a first atomic model that would include a subatomic particle.GCSE; Edexcel; Atomic structure - Edexcel Developing models of atoms. Dalton based his theory on the law of conservation . • The atom is mostly space: • Average Nuclear Diameter ~ 10-13 cm.In the third part of Dalton's atomic theory, he proposed that compounds are combinations of two or more different types of atoms. Second, the atoms of a given element are all exactly the same in structure and properties.
![Various atomic models [13] | Download Scientific Diagram](https://www.researchgate.net/publication/257386620/figure/fig1/AS:297615947255814@1447968579383/Various-atomic-models-13.png)
However, the most familiar force in our everyday lives, gravity, is not part of the Standard Model, as fitting gravity comfortably into this framework has proved to be a difficult . While atoms of same element has same . Neil Bohr’s model of the atom- Planetary model.
Rutherford model
Atomic Models • Conclusions of Rutherford Model • The atom consists of two parts, a dense indivisible nucleus and a diffuse electron cloud of negatively charged particles.
What are the Different Kinds of Models of Atoms?
The history of atomic chemistry. In this model, the black cloud represents the volume of space where electrons are likely to be found. His theory consists of five postulates.There are five basic atomic models which have contributed the structure of the atom itself. Atomic Structure. Rutherford Atomic Model. In this model, the atom is made up of negative electrons that float in a “soup” of positive charge, much like plums in a pudding or raisins in a fruit cake ( Figure 4. The Bohr Atomic Model, proposed by Niels Bohr in 1913, built upon Rutherford's findings and introduced new concepts to explain atomic structure. Bohr's model incorporated the following key attributes: Electrons orbit the nucleus in specific energy levels or shells.5 Electron Cloud . Atomic Theory through the Nineteenth Century.According to the accepted atomic model (Quantum Model), in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists expected that all of the alpha particles would pass through the gold foil with only a slight deflection or none at all. Ernest Rutherford, a New Zealand-born physicist, conducted the famous gold foil experiment in 1911.1: Atomic Models of the Twentieth Century.Atomic Models: Rutherford & Bohr | Introduction to Solid State Chemistry | Materials Science and Engineering | MIT OpenCourseWare. The first, sodium, is a highly reactive metal. Explore the experimental evidence, theoretical . What was thought of as a single particle about 1 × . He observed that a majority of the α-particles passed through the gold foil without any deflection. The numbers of particles in an atom can be calculated from its atomic number and mass number.The Standard Model includes the electromagnetic, strong and weak forces and all their carrier particles, and explains well how these forces act on all of the matter particles. A small fraction (1 in 100,000) were deflected nearly 180°. Students analyze conceptual, mathematical, and physical models of atoms. The Bohr model represents these energy levels as .This graphic takes a look at the key models proposed for the atom, and how they changed over time.
Atomic models
Bohr Atomic Model.An atomic model is a theory trying to explain the structure of the atom. Rutherford’s atomic model is a significant contribution to the field of atomic physics. Discovery of the Electron. Here are the different types of atomic model: Democritus, Dalton, Thompson, Rutherford, Nagaoka, Bohr, Sommerfeld, Schrodinger and Quantum Mechanics: 1. Thomson produced a visible beam in a cathode ray tube.Models of the atom - AQA Developing the atom.