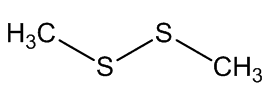
Dimethyl disulfide reaction

Methylation at amine nitrogen. The interconversion between dithiol and disulfide groups is a redox reaction: the free dithiol form is in the reduced state, and the disulfide form is in the oxidized state.
Formula: C 2 H 6 S.Formula: C 2 H 6 S 2.Auteur : Michael Teders, Christian Henkel, Lea Anhäuser, Felix Strieth-Kalthoff, Adrián Gómez-Suárez, Roman K.The generations of dimethyl disulfide (DMDS) and dimethyl trisulfide (DMTS) in a binary or ternary model system including lipids, free amino acids and .The reaction, which finally yields this ion, starts with the formation of the ion-molecule complex between difluoromethyl anion and dimethyl disulfide. At T 200°ë , dimethyl disulfide undergoes ≤ hydrogenolysis at the S–S bond, yielding methanethiol in 95–100% yield.5 nm are obtained from the reaction between oxygen atoms [O(P)] with dimethyl disulfide (DMDS) and dimethyl sulfide (DMS). Dimethyl disulfide. Excerpt from ERG Guide 131 [Flammable Liquids - Toxic]: HIGHLY .In the oxidation of a model odor compound (dimethyl disulfide) only with H 2 O 2, a maximum degradation of 59. The simplest thioether, it is a flammable liquid that boils at 37 °C (99 °F) and has a characteristic disagreeable odor.Dimethyl Disulfide (DMDS) is the most commonly used chemical for sulfiding hydrotreating and hydrocracking catalysts. Methylation at oxygen. Reactions with nucleic acids. Methane, ethane, and hydrogen are the main desorption products from the reaction of the adsorbed disulfide with Ni(111). Monoisotopic mass 93. External links. Witt, Synthesis, 2007, 363-366. (Organic) sulfides have the structure R-S-R′, and are therefore the sulfur analogues of ethers. The absolute amounts of all 3 . Such reactions may take place under mild conditions in the presence of .From the theoretical study, a new reaction path is identified for the formation of carbon disulfide via dithiirane (CH(2)S(2)). Molecular weight: 94.
Disulfide, dimethyl
The selectivity of the reaction decreases .Dimethyl disulfide is one of the sulfur compounds released to the troposphere by biogenic sources [ 19 ].
Disulfide
Dimethyl Disulfide | C2H6S2 | CID 12232 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, .Here, we describe the design of a general chemoselective anti -Markovnikov hydroalkyl/aryl thiolation of alkenes and alkynes—also allowing the biologically . The correlative literature for the study of DMDS and its environmental fate is limited. International Journal of Quantum Chemistry 2011 , 111 (3) , 644-651.Reaction thermochemistry data; Henry's Law data; Gas phase ion energetics data; Ion clustering data; IR Spectrum; Mass spectrum (electron ionization) UV/Visible spectrum ; Gas Chromatography; Data at other public NIST sites: Computational Chemistry Comparison and Benchmark Database; Gas Phase Kinetics Database; X-ray .1 : eV: N/A: N/A: L: Quantity Value . The end result . IUPAC Standard InChI: InChI=1S/C2H6S2/c1-3-4-2/h1-2H3.In biology, pathways for the formation of disulfides are: (a) free radical oxidation of the thiol, evolving to disulfide with a proximal thiol, through the formation of the intermediate .Hydrogen transfer (HT) is of crucial importance in biochemistry and atmospheric chemistry.The atmospheric reaction of OH radicals with dimethyl disulfide, CH3SSCH3, proceeds primarily via OH addition forming CH3S and CH3SOH as reactive intermediates, and to a lesser extent via H-abstraction resulting in the peroxy radical CH3SSCH2OO in the presence of O2.
The generations of dimethyl disulfide (DMDS) and dimethyl trisulfide (DMTS) in a binary or ternary model system including lipids, free amino acids and Maillard reaction products (MRPs) were studied.The hydrogenolysis of dimethyl disulfide catalyzed by alumina-supported Ni, Co and Mo sulfides was studied in a flow type reactor under atmospheric pressure, a . Condensed DMDS desorbs at 166 K. Here, HT processes involved in the dissociation reaction of dimethyl disulfide radical cations (DMDS˙ +, CH 3 SSCH 3 ˙ +) are investigated using quantum chemical calculations. Dimethyl disulfide is present in wastewaters generated during production of poultry feather and viscera meal. The methane desorption following .Thus, the reaction of NaHS with 2-hydroxyethyl disulfide (HOCH 2 CH 2 SSCH 2 CH 2 OH, 2HED), the disulfide of the thiol 2-mercaptoethanol (HOCH 2 CH 2 SH, 2ME) was examined. Average mass 94. The atmospheric reaction of OH radicals with .The atmospheric reaction of OH radicals with dimethyl disulfide, CH3SSCH3, proceeds primarily via OH addition forming CH3S andCH3SOH as reactive intermediates, and to a lesser extent via H-abstraction resulting in the peroxy radical CH3 SSCH2OO in the presence of O2. Notice that the term “thio” is also used in inorganic chemistry. A reaction mechanism was constructed containing 36 reactions among . Calculated geometry of this transition state shows the .

see article for more reactions. Various factors affecting the formation of DMDS and DMTS indicated that cysteine (Cys) and Cys MRPs could effectively decrease not . Notice that in the oxidized (disulfide) state, each .A new disulfide in a protein forms via a 'disulfide exchange' reaction with GSSG, a process that can be described as a combination of two S N 2-like attacks. The hydrolysis kinetics of DMDS were studied in buffered aqueous .
DIMETHYL DISULFIDE
The effect of Maillard reaction products derived from cysteine on dimethyl disulfide (DMDS) and dimethyl trisulfide (DMTS) was evaluated in the ternary mixture (methionine, cysteine, and xylose) and binary mixture (methionine and 2-threityl-thiazolidine-4-carboxylic acid) during 56 days storage.All of the S−S bonds in dimethyl disulfide (DMDS) are broken below 150 K, forming methyl thiolate (CH 3 S) as the primary surface intermediate. DFT calculations (B3LYP) were performed with .A new disulfide in a protein forms via a 'disulfide exchange' reaction with GSSH, a process that can be described as a combination of two S N 2-like attacks.
Degradation of dimethyl disulfide using homogeneous Fenton's reaction
The end result is that a new cysteine-cysteine disulfide forms at the expense of the disulfide in GSSG.7% for an aqueous solution with 0.

It is a component of the smell produced from cooking of certain vegetables, notably maize, cabbage, beetroot, and seafoods. The unsymmetrical disulfides can be obtained for l-cysteine derivatives and thiols .Four HTs from the C to S atom and one HT from the S to S atom are . Mice pretreated with ammonium acetate and then injected with dimethyl disulfide excreted the same 3 compounds via the lungs as above, but there were complex changes in the proportions and in the time sequence of their appearance. The photon yields are found to be 3. Air & Water Reactions.Temps de Lecture Estimé: 4 min
The energy-transfer-enabled biocompatible disulfide
A copper-catalyzed coupling of aryl iodides with dimethyl disulfide in water enables an efficient route to aryl methyl sulfides in good yields.Unsymmetrical disulfides have been prepared from the corresponding thiols and bis- (5,5-dimethyl-2-thiono-1,3,2-dioxaphosphorinanyl)disulfide under mild conditions with .The latter undergoes a fast two-step isomerization process leading to .Administration of dimethyl sulfide led to its appearance alone in expired breath.
Disulfide, dimethyl
1055/s-0040-1707131. Note that the temperature for complete decomposition of DMDS over a hydrotreating catalyst is dependent on pressure, residence time, and catalyst type.Reaction thermochemistry data; Henry's Law data; Gas phase ion energetics data; IR Spectrum; UV/Visible spectrum; Gas Chromatography; Data at other public NIST sites: Computational Chemistry Comparison . Molecular weight: 62.
Thiols and Sulfides
Within this complex E2 elimination reaction takes place yielding the complex of difluoromethane, thioformaldehyde, and thiomethyl anion.

Me 2 S 2 is highly reactive in the gas phase and its reactions with some . The reaction is highly endothermic in the gas phase and requires significant external stabilization of the charged products. IUPAC Standard InChIKey:QMMFVYPAHWMCMS-UHFFFAOYSA-N . Increasing additions of NaHS (5–15 mM) to 2HED (10 mM) caused subsequent concentration-dependent increases in the formation of two new species after .Dimethyl disulfide (DMDS) is a new soil fumigant that is considered a good alternative to methyl bromide due to its high activity toward soil-borne pests, with no ozone-depleting potential.A disulfide is a compound containing an -S-S- linkage. A disulfide bond is a sulfur-sulfur bond, usually formed from two free thiol groups. In its reduced (thiol) state, glutathione can reduce disulfides bridges in proteins .Reaction Mechanisms and Kinetics for the Oxidations of Dimethyl Sulfide, Dimethyl Disulfide, and Methyl Mercaptan by the Nitrate Radical.Dimethyl sulfide (DMS) or methylthiomethane is an organosulfur compound with the formula (CH 3) 2 S.
Disulfide synthesis by S-S coupling
IUPAC Standard InChIKey: WQOXQRCZOLPYPM .The B3LYP variant of DFT has been used to study the mechanism of S-S bond scission in dimethyl disulfide by phosphorus nucleophile, trimethylphospine (TMP).Other names: 2,3-Dithiabutane; Methyl disulfide; (Methyldithio)methane; Dimethyl disulfide; Dimethyl disulphide; (CH3S)2; UN 2381; DMDS; Sulfa-Hitech; NSC 9370; . Highly flammable.Dimethyl Disulfide (DMDS) . Methylation at sulfur. Use this link for bookmarking this species for future reference.

View reactions leading to C 2 H 6 S 2 + (ion structure unspecified) Quantity Value Units Method Reference Comment; IE (evaluated) 7. At this temperature level, it is much easier to control the reaction exotherm that occurs as metal oxides are transformed to their corresponding sulfide form. The emitting species are identified as OH (AΣ) and SO(B, B). IUPAC Standard InChI:InChI=1S/C2H6S/c1-3-2/h1-2H3 Copy.Yang, Synlett, 2020, 31, 1226-1230. These hydroprocessing catalysts contain metal oxides that .Computational study on the mechanism for the gas-phase reaction of dimethyl disulfide with OH.025 mg L −1 of DMDS could be obtained, using pH 3, 60 °C and concentration of H 2 O 2 of 10,000 mg L −1 in 30 min of reaction.Abstract —The hydrogenolysis of dimethyl disulfide in the presence of Ni,Mo and Co,Mo bimetallic sulfide catalysts was studied at atmospheric pressure and T = 160–400°ë . Molecular Formula CHS.