Distillation of alcohols

The process is applied to remove CO 2 for LPG separation or where it is desired to .Distillation separates the alcohol in a fermented beverage from the water and other non-alcohol byproducts suspended in the original solution. First Online: 01 January 2014. In the case of the formation of carboxylic acids, the . This process is so easy to do, that it only takes a short space to explain it. Each time a whiskey is heated, condensed, and collected, we call that a distillation. Distillation of binary mixture (ethanol-water) Spirits mainly consist of the ethanol and water in quite equal portions. Larger, more complicated alcohols are often isolated from volatile oils of plants by the process of steam distillation.
Alcohol production: downstream processes
If milky, the distillation can be ceased when the distillate is clear. The reaction is reversible.4) Amyl Alcohol is a group of eight related alcohol compounds. The distillation of mixtures to separate and purify substances is an ancient technique, and one that underpins modern society.Regarder la vidéoThis further video from ASFC Chemistry provides a summary overview of the differences between distillation and reflux and the chemistry of oxidation for both primary and secondary alcohols.Oxidation of ethanol by acidified K2Cr2O7 to form an aldehyde by distillation. As mentioned above, the difference in the boiling points of alcohol and water is utilized in distillation to separate these liquids from each other. [26] [55] The works of Taddeo Alderotti (1223–1296) describe a method for concentrating alcohol involving repeated fractional distillation through a water-cooled still, by which an . 212 F), distillers can evaporate the alcohol (mostly) by itself, collect the vapors into a tube and use cold .
Alcohol Distillation 101
However, often there may be other components present that although they may differ in relative volatility, are nevertheless volatile themselves.
The distillation
This process purifies and concentrates the remaining alcohol, which . First, we start with a fermented alcohol beverage — in this case, we’ll . Alcohol is the left over waste product from yeast breaking down complex sugars for their use as fuel.Alcohol - Fermentation, Synthesis, Distillation: The common sources of methanol, ethanol, and isopropyl alcohol have been discussed above.Spiritueux
Distilled spirit
Some distillate may be given off below this temperature - .
Alcohol distillation: basic principles and equipment
Separating the alcohol from the water and other components in the beer, generally by distillation, to get pure enough alcohol for use as fuel.
Download book EPUB. Let's now consider the two component system you will be using in the distillations you will perform .
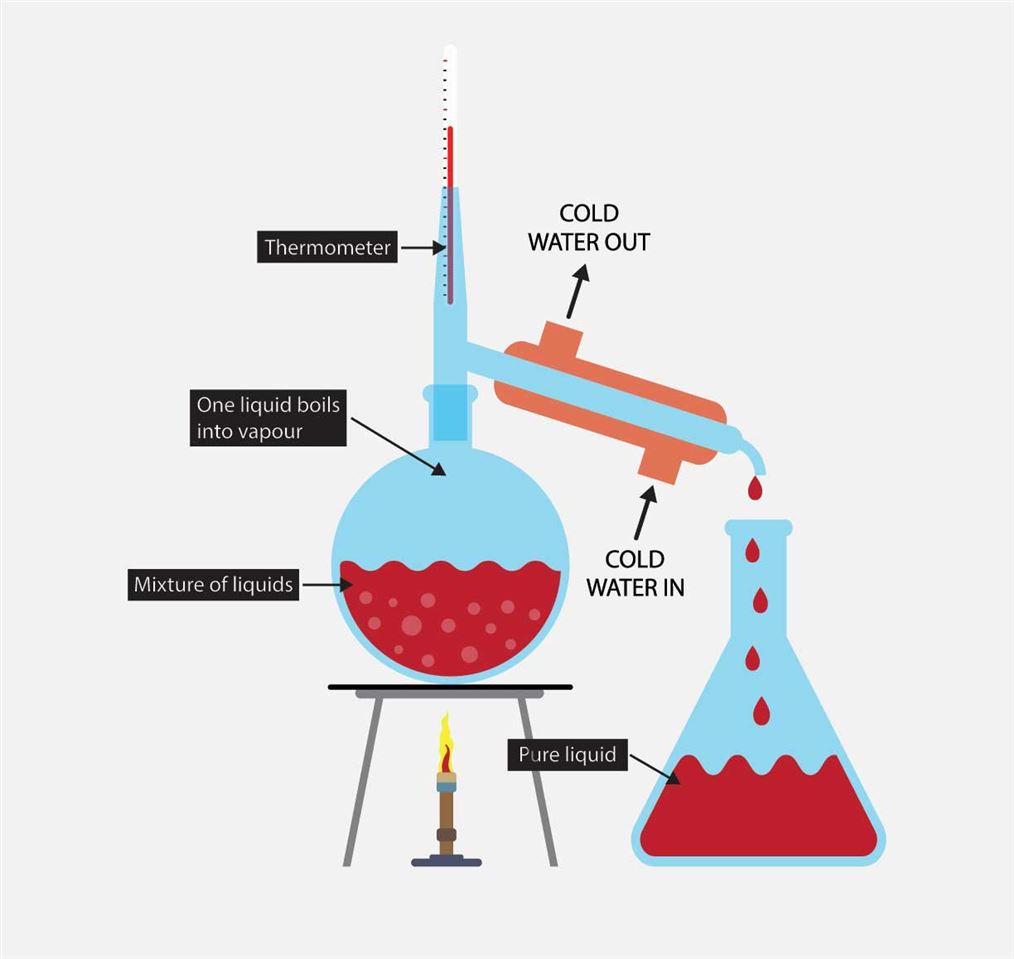
Distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part.Date de publication : 7 nov. Only 5% of the ethene is converted into ethanol at each pass through the reactor. P yridinium chlorochromate (PCC) is a milder version of chromic acid.
Reflux and distillation
Collect the distillate at a rate of 1 drop per second. It produces alcoholic drinks and the .
Distillation (video)
The process works because the boiling point of water is a higher temperature than that of alcohol. Heat water and dissolve as much sugar, either table sugar, sucrose, or molasses, etc, as . The base alcohol is heated, and certain parts of it are captured.
Distillation of alcohol
Describe the process and principles of . Many desalination plants . Distillation plays an important role in many water purification techniques. Processus visant à extraire les produits les plus volatils par évaporation puis condensation, la .Distillation is used to separate liquids from nonvolatile solids, as in the separation of alcoholic liquors from fermented materials, or in the separation of two or .Distillation is the concentration of alcohol and flavor/aroma compounds using evaporation.Elaboration du whisky. Collect the hearts until 91 °C (196 °F) steam temperature, after that you can . It produces alcoholic drinks and the fuels that power our .Distillation processes uses cryogenic distillation to remove acid gases from a gas stream. The pure alcohol is then diluted with water to 40% and used .Elimination Reactions.The process of creating ethanol, or drinking alcohol, is incredibly simple. If the liquid has too high of an alcohol content distillers can also add water to dilute the spirit and achieve a lower desired alcohol percentage. Fermenting grain (cooking it in water and treating it with enzymes to break down the starch and convert it to sugars) results in a 5-10% alcohol content. The distillate may appear cloudy, or a second layer may form on the top.
Distillation separation is a physical process based on the boiling points or relative volatility differences [68]. After the base alcohol is made, the next, and most crucial, step to making spirits is distillation. The catalyst used is solid silicon dioxide coated with phosphoric (V) acid. Natural products.

In the case of the formation of carboxylic acids, the alcohol is first oxidised to an aldehyde which is then oxidised further to the acid. In a typical distillation process, the vapor-liquid . Do it twice and call it a .Alcohol - Fermentation, Synthesis, Distillation | Britannica. The reaction between an alcohol and carboxylic acid to form an ester .
Distillation and the Isolation of Alcohol
La distillation, ce procédé qui existe depuis la nuit des temps, permet d’obtenir des spiritueux.An aldehyde is obtained if an excess amount of the alcohol is used, and the aldehyde is distilled off as soon as it forms. This process is fractional distillation, a scientific procedure which can be guaranteed to produce a perfect product every time --- a crystal clear alcohol of almost pharmaceutical quality. Cholesterol, found in .An alcohol is an organic compound with a hydroxyl (OH) functional group on an aliphatic carbon atom. Expected Learning Outcomes.
Distilled spirit
Excess hot, concentrated sulfuric acid or phosphoric acid is used as a catalyst. Ethanoic (acetic) acid is a useful solvent for such .
The Manufacture of Alcohols
It has the smell of . Tout savoir sur la distillation de l’alcool. Les alcools Vivant, rares, bio s et sans additifs, vous révèlent quelques . Various heterogeneously catalyzed processes have been developed in previous works operating at a high reaction .Alcohol distillation process: Basics of distilling with sugar wash & reflux stills.The process of distillation spread from the Middle East to Italy, where evidence of the distillation of alcohol appears from the School of Salerno in the 12th century. Home Science Chemistry. This whole concept works due to volatility, which roughly means, how easily it evaporates.

BEGINNERS GUIDE TO HOME DISTILLING - YouTubeyoutube.The Distillation.Chromic Acid Oxidation. Un ingénieux procédé physico-chimique qui peut sembler un peu mystérieux voire magique.An alcohol distillery.Heat the water with a Bunsen burner to create steam directly.

To improve the separation in a distillation, chemists often use a fractionating column, which . An excess of the alcohol means that there is not enough . Don’t forget to separate the heads (foreshot).Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points.This kind of distillation produces the most intense taste and smell, more than double distilled alcohol. The plant material is boiled in water, and the volatile oils are carried over by the steam, condensed, .alcohol, but instead of drinking the brew we subject it to a very rigorous purification process. Because OH is the functional group of all alcohols, we often represent alcohols by the general formula ROH, where R is an alkyl group. Distillation involves boiling the solution and then. Add steam indirectly through a steam line from the building. Some important applications of distillation are listed below. Cite this chapter.
The Basics of Distilling
In a distillation, a liquid is boiled in the . Primary alcohols can be oxidised to either aldehydes or carboxylic acids depending on the reaction conditions. Unlike chromic acid, PCC will not oxidize aldehydes to carboxylic acids. Alcohol vapour is passed over a hot (600 C) catalyst of aluminium oxide ( Al2O3) powder or pieces of porous pot. The level of knowledge you will need at the current level is . The whole idea is inspired by. Alcohol has a lower boiling point than water (78.Oxidation of 1o Alcohols with PCC to form Aldehydes. Distilling alcohol at home is a rewarding experience, combining science, patience, and artistry.comHow to Distill Alcohol : 7 Steps (with Pictures) - Instructablesinstructables. Basic distillation .Distillation and the Isolation of Alcohol.The distillation of a volatile material from non-volatile is one practical use of distillation which works very well.Most whiskey made in pot stills is either double distilled or triple distilled. All are toxic to some degree. 2015Temps de Lecture Estimé: 5 minBecause alcohol has a lower boiling point than water (173 F vs. Alcohols can also undergo dehydration to form alkenes. Laboratory oxidation of alcohols most often is carried out with chromic acid (H2CrO4) ( H 2 CrO 4), which usually is prepared as required from chromic oxide (CrO3) ( CrO 3) or from sodium dichromate (Na2Cr2O7) ( Na 2 Cr 2 O 7) in combination with sulfuric acid.The most commonly found alcohols are ethanol (C 2 H 5 OH, found in food and drink), methanol (CH 3 OH, found in copier toner), and 2-propanol (CH 3) 2 CHOH, commonly known as isopropyl alcohol).
Manquant :
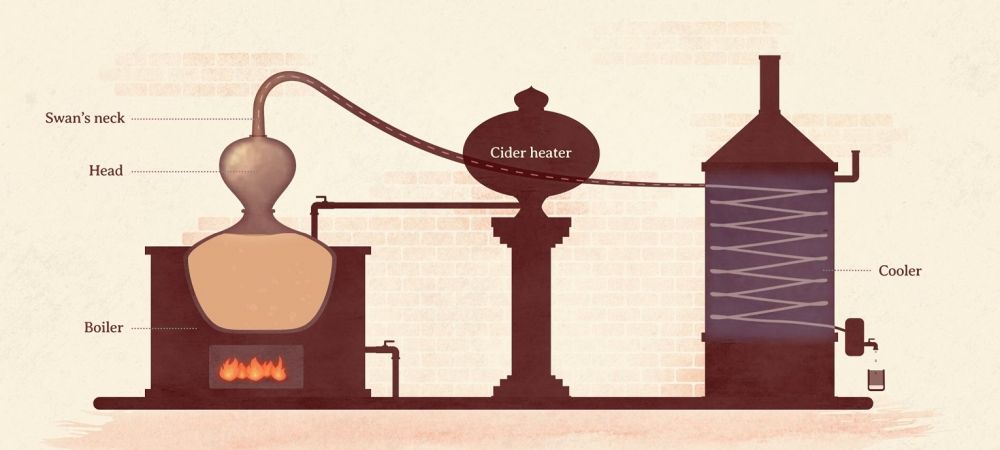
The still is the instrument that enables the alcohol steam to be collected and condensed to obtain a brandy .Home Distilling of Alcohol
By removing the ethanol from the equilibrium mixture and recycling .comRecommandé pour vous en fonction de ce qui est populaire • Avis
Théorie de la distillation des alcools
Evidence suggests that the distillation of alcohol was developed as far back as the 9th century.
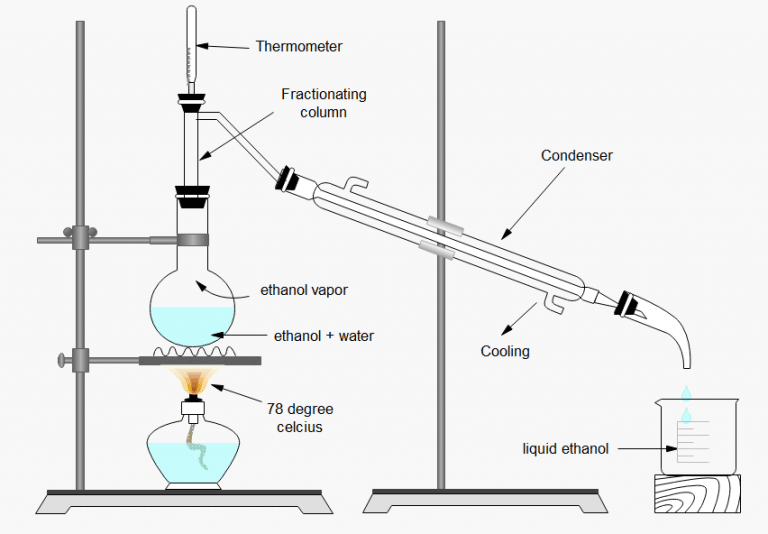
Alcohols have much higher boiling points than alkanes with the same number of carbon atoms.5°C compared to 100°C for water).
Tout savoir sur le processus de distillation de l'alcool
Phosphoric (V) acid is used an alternative dehydrating agent.Primary alcohols.
oxidation of alcohols
5) Furfural is a major portion of the fusel alcohols produced in distillation. Most people are familiar with ethyl alcohol (ethanol), the active ingredient in . Download book PDF.If the liquid has a low alcohol content, distillers repeat the distillation process to raise the alcohol concentration.Distillation is the process by which a liquid is heated to create a vapor and then condensed back into a liquid again.Overview
How Distilling Works

Some of the amyl alcohol compounds have strong smells, and are used in perfumes or cosmetics to yield the scent of roses, carnations, and even bananas. To prepare ethanol (alcohol) through fermentation and purify this using simple distillation. Preparation of Ethanol Fermentation Sources of alcohols.Separation of Alcohol:-.Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more chemically discrete substances; the .The distillation of mixtures to separate and purify substances is an ancient technique, and one that underpins modern society. Partial oxidation to aldehydes.








