Electric conductivity of seawater
Heat was generated within the seawater using alternating electric current at frequency of 60 Hz, the range of electrical field strength was 6.
thermal gradients, marine sediments are typically very conductive.

Calibration of standard seawater in electrical conductivity | Semantic Scholar. It represents a material's ability to conduct electric current.Because spatio-temporal variations in ocean heat content (OHC) are strongly predicted by ocean conductivity content (OCC) over most of the global ocean, we analyze the .

For this reason, . The range of conductivity . Tyler, Robert H. This ability is directly related to the concentration of ions in the water 1. Low levels of salts are found . Measurements of some . Numerical modeling and measurements are combined to estimate conductivity.The oceans play a special role in electromagnetic induction, due to their relatively high conductivity and the dynamo effect of ocean currents.
Electronic Conductivity
Ohmic heating can be .
Water quality standards
Temperature and Salinity.The conductivity sensor is unable to detect non-ionized constituents, leading to potential errors in salinity measurements. Additionally, due to penetration of the .The standard seawater solutions are either seawater from a particular place, or a standard KCl solution made in the laboratory.
Grayver
Electrical conductivity of the global ocean
The electrical conductivity of all the samples was measured using a standard conductance meter at temperatures between -2 ø and 30øC.It is found that the TEOS-10 salinity algorithm can be used without other corrections to estimate seawater salinities from electrical conductivity for all . Because the ocean .Measurements of the effect of pressure on the electrical conductivity of seawater were made for salinities of 2, 14, 22, and 35\permil over pressure and temperature ranges of 0 . method predicts closer electrical conductivity values to the experimental data of , while the Pawlowicz model has lower values.
Conductivity (electrolytic)
Electronic conductivity in anode materials is considered as the primary selec- tion criterion, with an absolute minimum of 1 S cm − 1 .
Ocean Electromagnetics
Electrical conductivity is measured in microsiemens per centimeter (uS/cm).The average vertical electrical conductance of the world ocean is ∼12,000 S with local values reaching up to 30,000 S. Using the formula provided earlier, the .5 S/m in cold deep waters, and approaches 6 S/m in . In contrast, the optical refractive index of seawater is sensitive to both non-ionized and ionized solutes.
Seawater: Composition and Properties
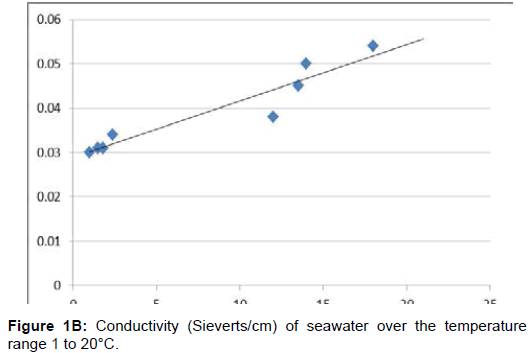
S = 35000 mg/l = 35 ‰. The electrical conductivity values estimated by McCleskey are the most accurate within a difference of 0. Conductivity for liquid seawater Figure 1B increases as a function of temperature over the range 0 to 30°C. Thus, electrons flow like a “sea of electrons” through metals.Electrical Conductivity of Seawater.The global ocean is a prominent electrical conductor with a relatively well-known geometry. The increase in conductivity is a linear dependence on temperature for the aqueous solution and . Imagine a scenario where an oceanographer needs to calculate the salinity of seawater with an electrical conductivity of 4 S/m at a temperature of 20°C.0000\permil has been evaluated.include electrical conductivity, colour, pH, apparent speci fic heat and density of the seawater were measured. In sea water free state of ions are plenty available.The concentration of the KCl solution whose conductivity is that of standard seawater (35 ‰) at 15°C.Seawater battery is an advanced energy storage system that enables conversion of chemical energy to electricity by consuming metals, dissolved oxygen and . An alternating voltage is generally used in order to minimize water electrolysis. Freezing Point of Seawater vs. To interpret a chemical reaction by observing aqueous solution conductivity.6 (mS/cm) and strongly dependent on TDS and temperature. The latter provides greater accuracy and has recently become the standard. The variation of the. With the original samples under strict control our intention was to study the effect of the organic content, storage time, and stabilizing upon the con- ductivity and permittivity.5, 1, 2, 4, 8, 16, 32, 48, and 72 h), using a Thermo Orion 3-star pH meter and WTW EC .Electrical conductivity of seawater. [citation needed] The resistance is measured by a conductivity meter. If sea water is mixed with fresh water aquifer than . The influence of urea dissolved in seawater on the .
Electrical Conductivity
KLEIN* (Received 28 June 1976; in revised form 13 December 1976; accepted 4 January 1977) Abstract--A new method of measuring the variation with . In comparison, seawater, with 2. Zweng, James R. An interesting fact is that as water warms, its conductivity increases.As conductivity is temperature-dependent and conductivity can be used to determine salinity, oceanographers measure conductivity, temperature, and depth (CTD) when studying seawater.Auteur : Robert H.A typical temperature dependence data set for seawater from -20 to 18°C is shown in Figure 1, where seawater freezes at -2°C.Here, we lay out our logic and method of creating a linear equation that allows temperature-compensated conductivity sensors to estimate salinity of surface seawater (practical salinity values of 22-42 at 0-200m depth and 5-35°C) within 5% of the true value. IEEE Journal of Oceanic Engineering. The precision of
Why Do Oceanographers Measure The Conductivity Of Seawater?
The pH of seawater ranges from 7.Compilation of 3D global conductivity model of the Earth for .
(PDF) Electrical Conductivity of Seawater
Practical salinity, which was introduced with the PSS-78 standard as the measure of the salinity of seawater, is calculated from the ratio K 15 of the electrical .1 Electronic/ionic conductivity.When dealing with any sample of seawater, the primary information required is usually a measurement of the salinity.Electrical conductivity is the number of ions present in water.Electrical conductivity (EC) and pH of the EICP solutions were measured at different time intervals (0, 0.
Properties of seawater
The average vertical electrical conductance of the world ocean is Additionally, due to penetration of the seawater and ∼12,000 S with local values reaching up to 30,000 S. The density is obtained from the salinity and the temperature, as are the .Electrical conductivity (EC) refers to a solution’s ability to carry an electrical current, measured in microsiemens per centimeter (µS/cm). In this article, a refractometer based on a V-shaped groove was established.The effects of temperature, electrical field strength and the concentrations of total dissolved solids (TDS) on electrical conductivity during ohmic heating of seawater were investigated.5–6 S m −1, is a very good .McCleskey et al.Electric conductivity profiles show similar vertical structures at all locations.The electrical conductivity of seawater ranges from 44,000 to 58,000 µmhos/cm (or µS/cm) with an average of about 50,000 µmhos/cm. Environmental Science. The range of conductivity during ohmic heating was 55–399.This table serves as a quick reference for those exploring conductivity and salinity-related topics. Freshwater is usually between 0 and 1,500 uS/cm and typical sea water has a conductivity value of about 50,000 uS/cm. Corpus ID: 95152996.

Of course, conductivity and salinity are highly and positively correlated.
Conductivity (EC) vs Total Dissolved Solids (TDS)
Electrical Properties of Sea Water: Theory and Applications
Pergamon Press.1016/0048-9697 (86)90230-5. September 2011. Electrical conductivity is based on the flow of electrons. These conductive ions come from dissolved salts and inorganic materials such as alkalis, . Example of Conductivity to Salinity Calculator. The samples with S<35\permil were prepared by . DAUPHINEE* and H. Typical crystalline rocks of the Earth's crust have electrical conductivities in the range of 10 –6 –10 –2 S m −1, (Siemen [1/Ω]).Experimental data of seawater electrical conductivity are used to compare the estimated electrical conductivity values from the methods and assess their accuracy.Since the ionic content of seawater controls its electrical conductivity, measurements of the latter are widely used as a proxy for this purpose. Oceanography is the study of the ocean, and oceanographers have a very important job in climate research.
Manquant :
seawaterAbstract: The ratios R_{s,t,o} of electrical conductivity of seawater samples of precisely known salinity to standard seawater at the same temperature have been measured over a wide range of salinities from 0 to 42\permil S and over the full range of oceanic temperatures from -2 to 35\deg C.Electrical conductivity is a measure of the saltiness of the water and is measured on a scale from 0 to 50,000 uS/cm.To even out that gradient, positively charged ions from seawater, such as sodium, flow through the system to the freshwater, increasing the pressure on the .69 may be used to convert conductivity to salinity for practical purposes. Boyer, Takuto Minami, Melissa M.Enhancing seawater electrical conductivity not only improves the propulsion efficiency and thrust density significantly, but also facilitates the miniatur-ization and lightweight of . Many properties, such as the concentrations of the conservative elements, can be calculated directly from the salinity, and others are related to it. Conference: Annual Meeting 2011 of the International . If you were to compare the conductivity of your everyday drinking water to the vastness of seawater, you would .The average vertical electrical conductance of the world ocean is Additionally, due to penetration of the seawater and ∼12,000 S with local values reaching up to 30,000 S. Seawater is an aqueous multi-component electrolyte with a range of electrical properties. Electric conductivity decreases with increasing depth first and then slowly increases . Calibration of standard . Electrical Conductivity of Seawater vs.Auteur : Zeyu Zheng, Yang Fu, Kaizhou Liu, Rui Xiao, Xiaohui Wang, Haibo Shi Density of Seawater vs.netRecommandé pour vous en fonction de ce qui est populaire • AvisThree-stage vertical distribution of seawater conductivity
The concentration of the potassium chloride solution (KCI) which has the same conductivity as 15\deg C at P79 standard seawater corrected to 35. Within the parameters of Earth’s oceans, the electrical conductivity of seawater depends on temperature, salinity, and .Auteur : Alexander V.Practical salinity, which was introduced with the PSS-78 standard as the measure of the salinity of seawater, is calculated from the ratio K 15 of the electrical conductivity of a seawater sample at a temperature of 15 °C and the pressure of a standard atmosphere and the conductivity value of a potassium chloride (KCl) solution .

Quality parameters include electrical conductivity, colour, pH, apparent specific heat and density of the seawater were measured at room temperature before and after the ohmic heating. When salts and other inorganic compounds break down in water, they transform into ions – which increase the conductivity.Deep-Sea Research, 1977, Vol. It is commonly signified by the Greek letter σ ( sigma ), but κ ( kappa) (especially in electrical engineering) and γ ( gamma) are sometimes used.Based on Conductivity–Temperature–Depth (CTD) and Lowered Acoustic Doppler Current Profiler (LADCP) observation conducted during the summer of 2022, .








-et-thomas-montagnier-directeur-regional-de-la-saur-(a-droite-au-1-e-plan)-ont-signe-le-contrat-photo-progres-francoise-sutour-1671126359.jpg)


