Electronic configuration of all elements
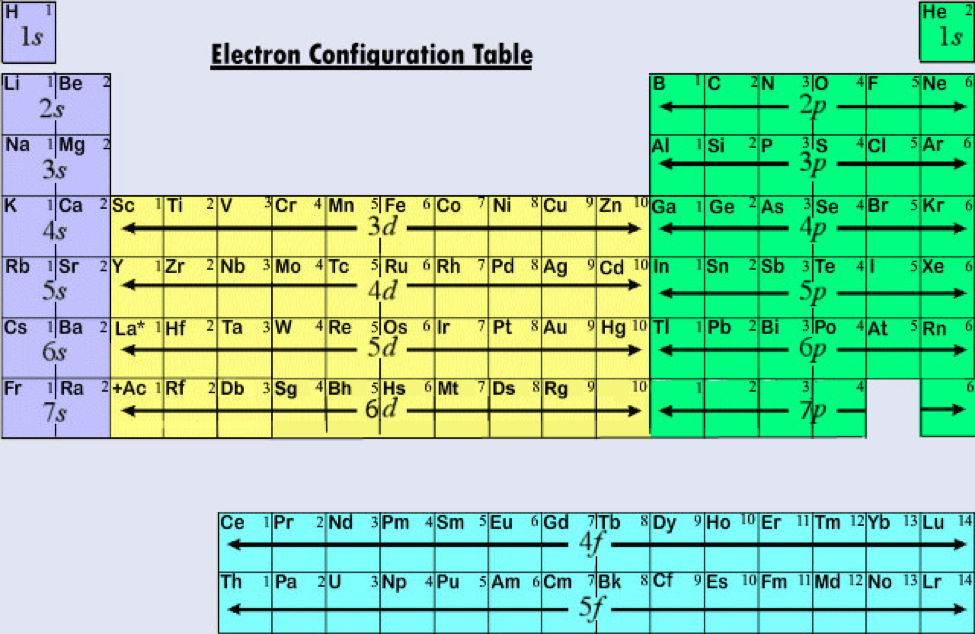
Specifically, an element’s position in the periodic table helps you figure out its electron configuration, how the electrons are organized around the nucleus .
First 30 Elements for Beginners: Mastering Electronic Configurations
Electron configurations of the elements (data page)
Liste alphabétique des éléments de configuration électronique
Generally, the electronic configuration of these elements is (n-1) d 1–10 ns 1–2. Explain the rules for filling electrons in atomic orbitals -- . Each method serves a distinct function and has its own set of disadvantages.
Electronic configuration
The potassium element has atomic number 19. of electrons, shells, etc.Inner transition elements are metallic elements in which the last electron added occupies an f orbital. There are two inner transition series: Describe how the number of valence electrons are related the arrangement of the main group elements and its chemical behavior.Il s'agit d'une liste alphabétique de configuration électronique de tous les éléments du tableau périodique. For example, the electron configuration of lithium, 1s²2s¹, tells us .Elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium).Notation de base de gaz noble utilisée. Bracketed noble gas symbols on the left represent inner configurations that are the same in each period.Electronic Configuration in Groups.An element's atomic number, which is equivalent to the number of protons in the atom's nucleus, determines the element's electronic configuration. Alors que la structure électronique des éléments plus .List of electrons configurations. It is a visual representation of the .Electron configuration. However, there is an exception for the d-block and f-block, in which the energy level, n for the d block is .This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. The electronic configuration of sodium, for example, is 1 s 2 2 s 2 2 p 6 3 s 1. Identify and explain exceptions to predicted electron configurations for atoms and ions.The electron configuration of an element describes how electrons are distributed in its atomic orbitals. University of Waterloo. The theoretical basis for the table in terms of atomic number and electron configuration does not allow for an unknown element between Sn and Sb. The d block includes the middle area marked by s and p blocks in the periodic table. 3s2 3p5 3d10 4s2 4p3 3s2 3p2 1s2 2s2 2p5 4d10 5s2 5p5 4s2 3d2 4s2 1s2 2s2 2p4 1s2 2s2 2p3.electronic configuration, the arrangement of electrons in orbitals around an atomic nucleus. While the electronic structure of the lighter elements is well-studied, once you get to the heavier man-made elements, these configurations are predicted or calculated based on periodic table trends. Dive into the world of. Note: although the third shell can hold up to 18 electrons, the filling of the shells follows a more complicated pattern after potassium and calcium. The position of each element in the table gives important information about its structure, properties, and behavior in chemical reactions.In the case of first row transition metals, the electron configuration would simply be [Ar] 4s x 3d x.For hydrogen, therefore, the single electron is placed in the 1 s orbital, which is the orbital lowest in energy (Figure 6.Note that these electron configurations are given for neutral atoms in the gas phase, which are not the same as the electron configurations for the same atoms in chemical .
Periodic table (electron configurations)
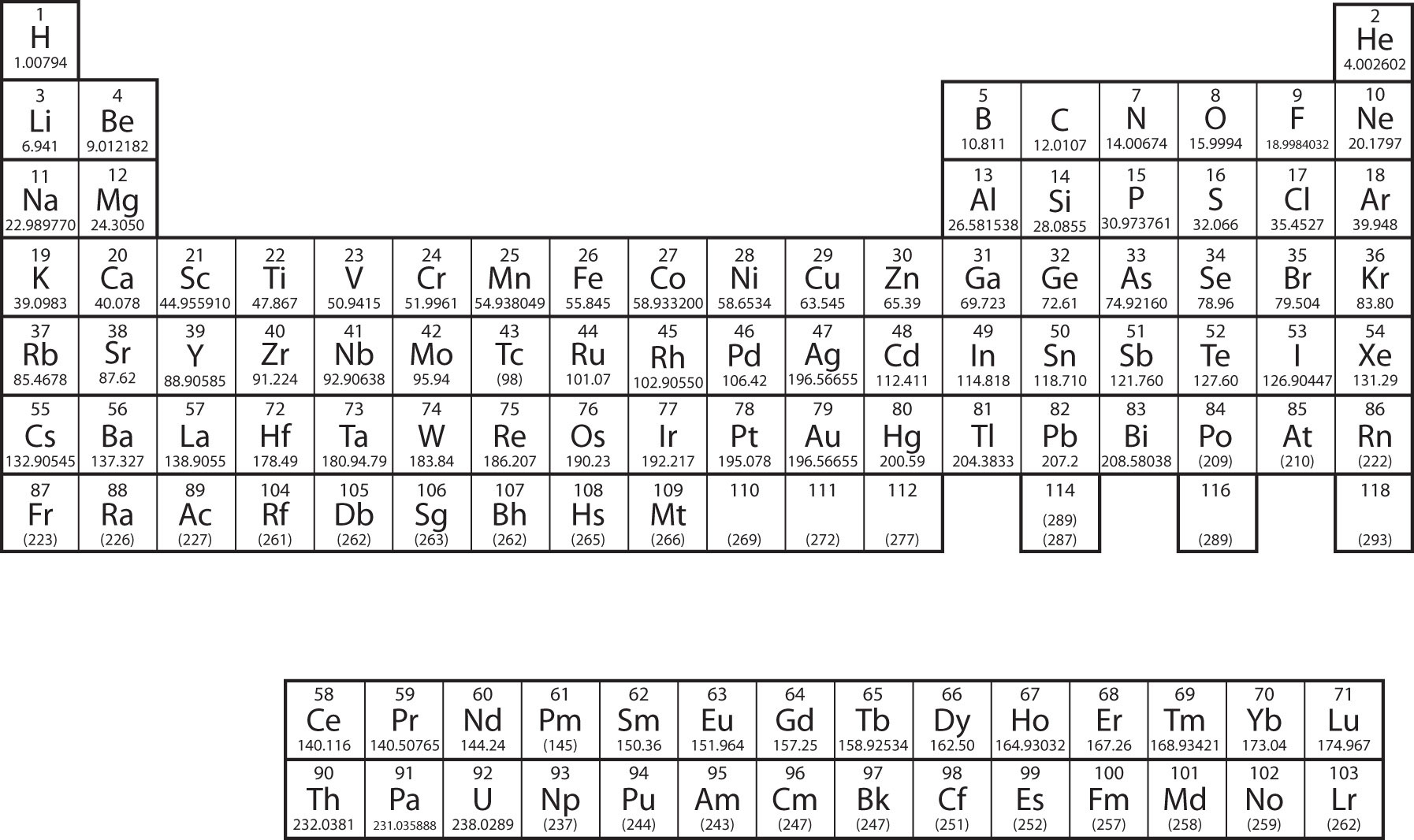
The organization of electrons in atoms explains not only the shape of the periodic table but also the fact that .
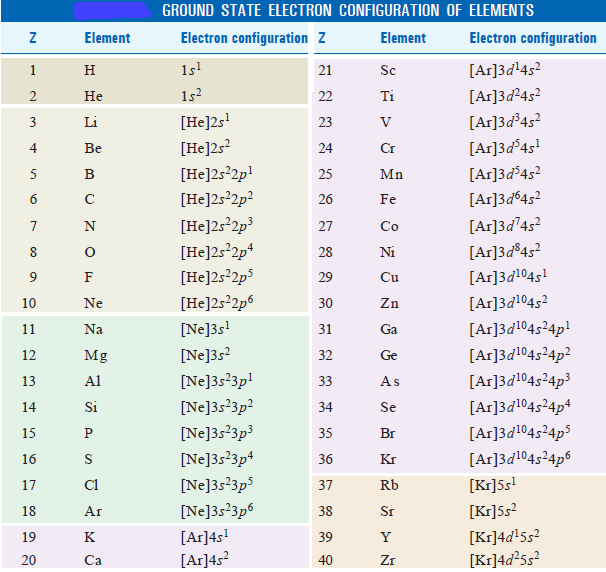
If there are more protons than electrons, an atomic ion has a positive charge and is called a cation. Since the inner electron level, 4f orbitals are successively filled from (4f 1 to 4f 14) though there are outer incompletely filled electron levels, these elements are called inner transition elements and the series are called inner transition series. A Bohr diagram of lithium. Electron configurations describe where electrons are located around the nucleus of an atom. The amount of electrons in each shell and subshell is typically represented by a sequence of numbers and letters, such as 1s 2s 2 2p 6 , when describing the electronic configuration of an atom.
Electronic Configuration of the d-block Elements
Instead, it’s more like a filing system.Electronic Configuration of Elements.

They all have a similar electron configuration in their valence shells: a single s electron. Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction. Grayed out electron numbers indicate subshells filled to their maximum.
Liste alphabétique des éléments de configuration électronique
Chung (Peter) Chieh.Full electron configuration for every element in the periodic table |118 elements| Welcome to our comprehensive guide on the electron configurations of all 118 elements in the periodic table.
Electron configuration
To correlate the arrangement of atoms in the periodic table results in blocks corresponding to filling of the ns, np, nd, and nf orbitals.The general electronic configuration of the Lanthanide element is. The energy level, n, can be determined based on the periodic table, simply by looking at the row number in which the element is in.This is an alphabetical electron configuration list of all the elements of the periodic table. In this article, we’ve organized all the elements of the periodic table by atomic number with their name and provided their full electron . Typically, you need at . The (n–1) remains for the inward d orbitals which may have one to ten electrons and the peripheral ns orbital may have one or two electrons.Regarder la vidéo5:08Introduction to electron configurations. Thus, the electron configuration and orbital diagram of lithium are: About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features NFL Sunday Ticket
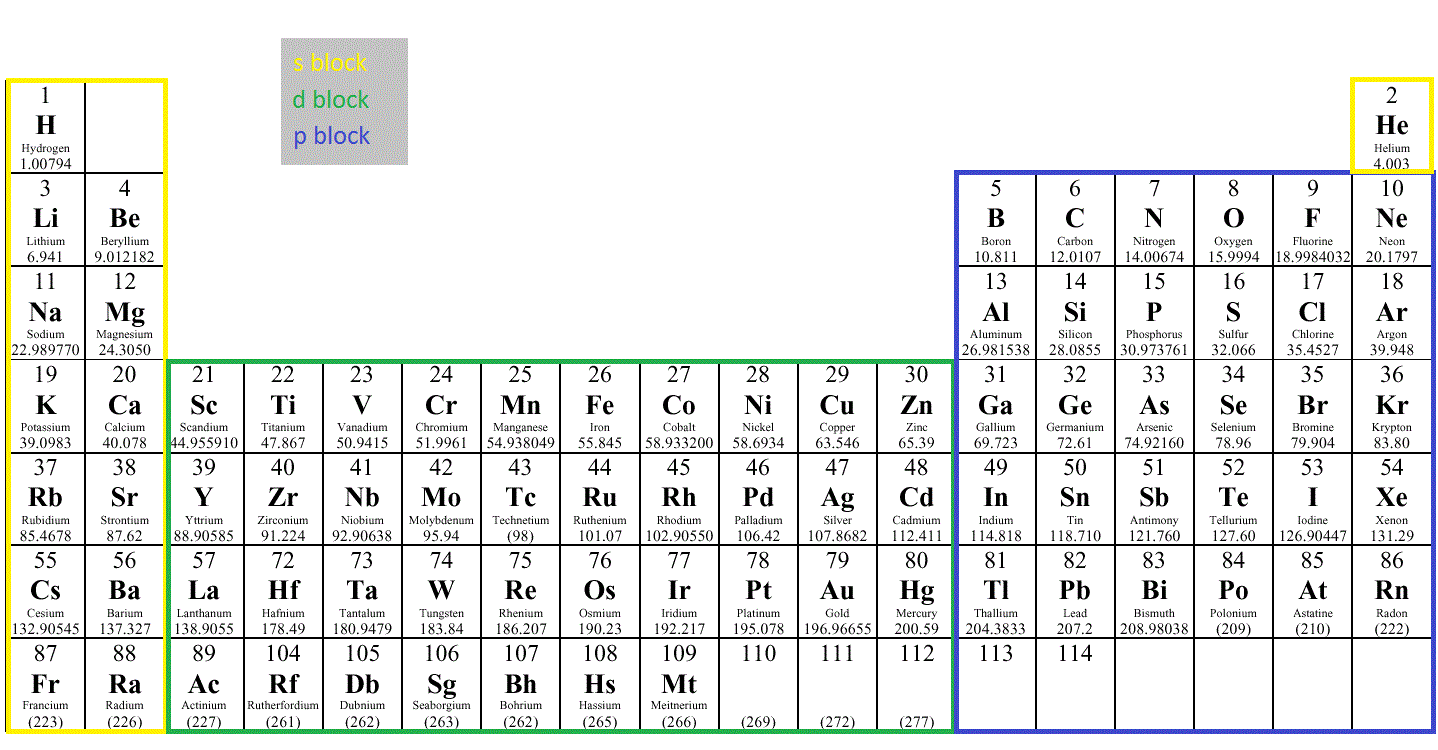
What are electron configurations?The electronic configurations for all of the elements in the periodic table can be written using the rules above.Periodic table (electron configurations) Configurations of elements 109 and above are not available.The electronic configurations of an atom follow a standard nomenclature in which all electron-containing atomic subshells are arranged in a sequence (with the number of electrons they possess written in superscript).
Electronic Configuration of Elements
Thus, we observe a similar .9: Electron Configurations and the Periodic Table. Elements that have .Temps de Lecture Estimé: 9 min The atomic number of oxygen is 8. And has 19 electrons . So, the abbreviated notation for argon would be [Ne] 3s²3p⁶. We place one electron in the orbital that is . Electron configurations are written using this methods: s p d f notation for orbital diagrams noble gas notation. If there are more electrons than protons, the ion has a negative charge and is called an anion. All three orbitals need to be drawn even if one or more is unoccupied. The valence shells of the inner transition elements consist of the ( n – 2) f, the ( n – 1) d, and the ns subshells.Unlock the secrets of chemistry with our beginner-friendly guide to mastering the electronic configurations of the first 30 elements.The electron configuration for phosphorus is 1s 2 2s 2 2p6 3 s2 3p3 and the orbital diagram is drawn below. They are shown in green in Figure 3.When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the . Orbital diagrams.The electronic configurations of the elements in group 1: The atoms of all group 1 elements have similar chemical properties and reactions because they all have one electron in their outer shell . It implies that an .4: Electron Configurations and Electronic Orbital Diagrams (Review) is shared under a CC BY-NC-SA 4. However, it's easy to determine the configuration of electrons for heavier elements by .
The periodic table, electron shells, and orbitals
Because much of the chemistry of an element is influenced by valence electrons, we would expect that these elements would have similar chemistry—and they do.

29 shows the lowest energy, or ground-state, electron configuration for these elements as well as that for atoms of each of the known elements. Learning Objectives.Déterminer les configurations électroniques des atomes à l'état fondamental prédites. For these two elements, the third shell holds 8 and the remaining electrons (for reasons of stability) occupy the fourth . [Xe] 4f1-14 5d10-1 6s2.

Its electronic configuration is 2,8; Electronic Configuration of Elements. On the other hand, the standard notation . 1 ), and the electron configuration is written as 1 s1 and read as “one-s-one. Electron configurations of atoms follow a standard notation in which all . Following the 2s sublevel is the 2p, and p sublevels always consist of three orbitals. Predictions from reliable sources have been used for these elements. Elements in the same group have the same number of electrons in their outermost shell leading to similar valence shell electronic configuration.Learn how to distribute electrons across different orbitals of an atom using the Aufbau principle. By convention, the ms = +1 2 m s = + 1 2 value is usually filled first.Derive the predicted ground-state electron configurations of atoms. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and . Electron Configuration of Some Other Common Elements.So, before drawing an electronic configuration we need to extract some information from the periodic table like the atomic number, no. Electron atomic and molecular orbitals.The first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum numbers as the two electrons in helium.Auteur : Sal Khan1 Elements are listed in the periodic table in an ordered, systematic way that correlates with a periodicity of their chemical and physical properties.
Electron configurations article (article)
See also: Periodic Table Cipher. The atoms of elements in the same vertical column . In doing so, we obtain three quantum numbers (n,l,m l), which are the same as the ones obtained from solving . However, it's easy to determine the configuration of electrons for . The remaining electron must occupy the orbital of next lowest energy, the 2 s orbital (Figure 5.One more point needs to be emphasized about the relationship between electron configuration and the periodic table.
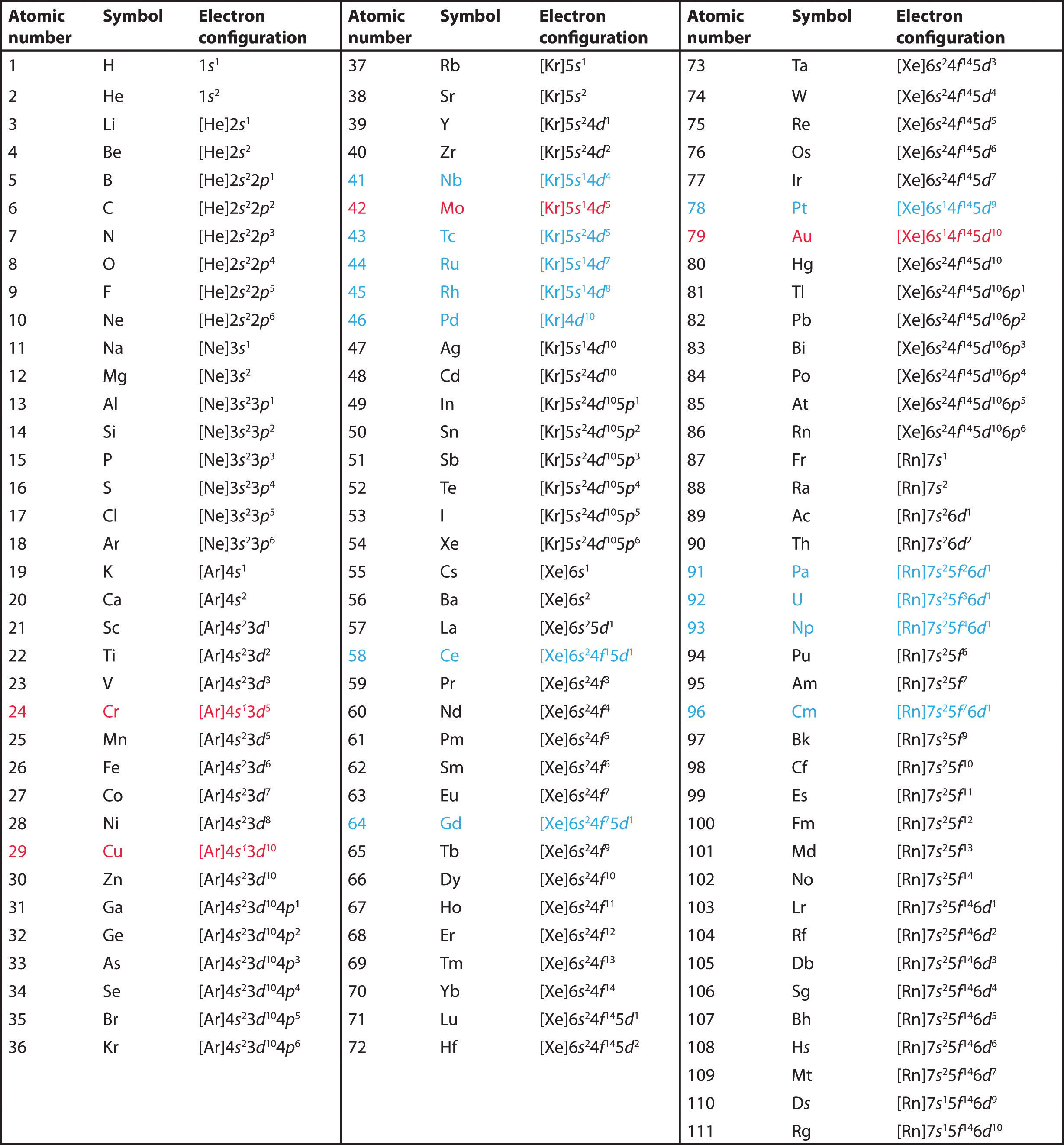
The electronic configuration of an atom in the quantum-mechanical . Ainsi, par exemple, le néon est écrit en utilisant ce raccourci comme [He]2s 2 2p 6 plutôt que 1s 2 2s 2 2p 6. In atomic physics and quantum chemistry, the electron configuration is the distribution of .











