Energy in chemical reactions ii

We begin our study of the energetics of chemical reactions with our understanding of mass .Balises :Energy in Chemical ReactionsChemical Reaction and EnergyThermodynamics
Energy Relationships in Chemical Reactions
energy required to break a chemical bond. Types of chemical reactions include synthesis, decomposition, replacement, and combustion reactions.The energy released in this nuclear reaction is more than 100,000 times greater than that of a typical chemical reaction, even though the decay of 14 C is a relatively low-energy nuclear reaction. What are some types of chemical reactions? Some chemical reactions can be very calm and boring, while other reactions release a great . Hot packs: Chemical reaction produces heat or thermal energy. Determine whether a reaction is endothermic or exothermic . The substances that go into a chemical reaction are called the reactants, and the substances produced at the end of the reaction are known as the products. If ΔS° and ΔH° for a reaction have the same sign, then the sign of ΔG° depends on the relative magnitudes of the ΔH° and TΔS° terms. Their work has focused on a class of chemical reactions important in the energy transition that involve adding oxygen to small organic (carbon-containing) . In other reactions we can observe this change when a reaction starts to give off light or when a reaction . A chemical reaction is a process in which some substances, called reactants, change into different substances, called products.These are not the only products of this reaction that .Chemical reactions often involve changes in energy due to the breaking and formation of bonds.0 (8 reviews) Get a hint.Quiz yourself with questions and answers for Energy in Chemical Reactions II Unit Test, so you can be ready for test day.4) Standard enthalpy of formation and reaction (6. The energy of the O=O bond is 498 kJ/mol.Chemical reactions.Balises :Energy and Chemical ReactionsLibreTextsChemical Reaction and Energy
Energy and Chemical Reactions#
new bonds are made in the. This concept is most easily understood . Our study of gases in Chapter 6, and our definition of .Chapter 5 Energy Changes in Chemical Reactions. Study with Quizlet and memorize flashcards containing terms like Use the reaction to complete the sentence.Balises :Energy in Chemical ReactionsEnergy and Chemical ReactionsQuizletEnergy in Chemical Reactions II Unit Test Flashcards | Quizlet. Cellular respiration: A set of reactions that changes the chemical energy in glucose into chemical energy in ATP, a .Balises :Energy in Chemical ReactionsEnergy and Chemical ReactionsThermodynamicsReaction RatesOur Sun, a Giant Nuclear Power Plant
Energy in Chemical Reactions II Unit Test
2H-H + O=O → 2H-O-H.4: Energy and Chemical Change - Chemistry LibreTextschem.Balises :Royal Society of ChemistryScienceTransferChemical energy Students also viewed.Balises :CreditMachine learningInterceptionMolecule
Manquant :
energyStudy with Quizlet and memorize flashcards containing terms like The combustion of glucose, C6 H12 O6 (s), produces carbon dioxide, CO2 (g), and water, H2 O(g), according to the equation below. Propane: Burned to produce heat and light.Balises :Royal Society of ChemistryChemical Society ReviewsTransferAtlanta0 license and was authored, remixed, and/or curated by Anonymous . (a) Without an enzyme, the energy input needed for a reaction to begin is high. Carbon monoxide and oxygen combine to produce carbon dioxide. Use the chemical equation and the bond diagram to answer the question.Balises :Energy in Chemical ReactionsChemistryReaction EnergyHonshucomPls help! 2CO + O2 → 2CO2 - Brainly.10: Energetics of Chemical Reactions.Balises :Energy and Chemical ReactionsLibreTextsHuman Biology These are exothermic reactions.Exothermic reactions may occur spontaneously and result in higher randomness or entropy (ΔS > 0) of the system.Balises :Royal Society of ChemistryReaction EnergyChemical Society Reviews During the reaction, chemical bonds break in the reactants and new chemical bonds form in the products. This can be used to classify reactions as exothermic or endothermic.3 Chemical Equations, you learned that applying a small amount of heat to solid ammonium dichromate initiates a vigorous reaction that produces chromium(III) oxide, nitrogen gas, and water vapor. Thermal energy Energy that results from atomic and molecular motion; . In endothermic reactions, more energy is absorbed when the bonds in the . The total bond energy of the products is 1,472 kJ. Burning charcoal to cook food on a grill is an example of an exothermic reaction. Bond-breaking is an endothermic process.Chapter 6 – Energy Relationships in Chemical Reactions. 2CO + O2 → 2CO2.Exergonic Reactions. Under most circumstances, particularly when the pressure and volume are kept constant, these changes can be ascribed to changes in enthalpy ΔH. (c) Lightning is an example of electrical energy, which is due to the flow of electrically charged .Chemists are concerned with both physical and chemical change, and the energy associated with each. In some reactions we see this as a change in the temperature.The water oxidation reaction, a crucial process for solar energy conversion, has garnered significant research attention. Chemical reactions that absorb (or use) energy are called endothermic.3: Energy Changes and Chemical Reactions | PPT - . So, like it or not, we need . ) per mole of glucose.The standard free-energy change can be calculated from the definition of free energy, if the standard enthalpy and entropy changes are known, using Equation 18.Energy in chemical reactions.The total chemical potential energy of the reactants is greater than the total chemical potential energy of the products. A chemical reaction that releases energy is called an exergonic reaction.Balises :Energy in Chemical ReactionsDegree CelsiusRotationcomRecommandé pour vous en fonction de ce qui est populaire • Avis In the reaction of hydrogen gas and oxygen gas to form water, the glowing splint. The total bond energy of the products is .The first example of intramolecular nucleophilic addition of 1,4-diazabutatriene to ester is disclosed.comEnergy in Chemical Reactions II Unit Test | Quizletquizlet. Terms in this set (14) Which event occurs during an endothermic reaction? Chemical reactions involve a redistribution of energy within the reacting chemicals and with their environment.Electrical energy is used rather than heat energy to carry out this reaction.
Energy in Chemical Reactions II Unit Test Flashcards
Energy in Chemical Reactions II Unit Test.This chapter introduces you to thermochemistry, a branch of chemistry that describes the energy changes that occur during chemical reactions. The total bond energy of the products is 1,472 KJ.Balises :Energy in Chemical ReactionsEnergy and Chemical ReactionsChemistry The energy of the O-H bond is 467 kJ/mol.All chemical reactions involve energy changes. Click the card to flip 👆. What Is Activation Energy? Understanding the role that energy plays within a reaction is a powerful way to predict how it will react.1) Energy changes in chemical reactions (6. In some situations, the energy .7: ΔG° = ΔH° − TΔS°.
Energy in Chemical Reactions I Unit Test Flashcards
The software powering a new web-based tool can recommend a diverse set of molecules (left) to test new reactions on.When chemical reactions occur, the energy changes are relatively modest and the mass changes are too small to measure, so the laws of conservation of matter and energy hold well. Complete the statements. In some reactions, we are able to observe these energy changes as either an increase or a decrease in the overall energy of the system.Many chemical reactions release energy in the form of heat, light, or sound.comCopy of Unit 4 Energy In Chemical Reactions II Study Guide 1 . However, in nuclear reactions, the energy changes are much larger (by factors of a million or so), the mass changes are measurable, and matter-energy conversions are . They are denoted by a negative heat flow (heat is lost to the surroundings) and decrease in enthalpy (ΔH < 0).

Cold packs: Chemical energy is absorbed in a reaction.Balises :Energy in Chemical ReactionsQuizletPractice9: Essential Skills 4 This page titled 5: Energy Changes in Chemical Reactions is shared under a CC BY-NC-SA 3.Now that we have shown how energy, work, and heat are related, we are ready to consider energy changes in chemical reactions. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Use the reaction to complete the sentence.All chemical reactions are accompanied by energy changes.Created 2 years ago. Learning Objectives. 2H2 + O2 → 2H2O. Energy is absorbed to break bonds.Unit 2 Test: Chemical Reactions and Energy Flashcards | .
Four C=O and four O-H bonds need to form.Chemical changes involve chemical reactions, in which some substances, called reactants, change at the molecular level to form new substances, called products.Understand: energy of chemical reactions.Balises :Energy in Chemical ReactionsEnergy and Chemical ReactionsLibreTexts
Chemical reactions for the energy transition
Chemical reactions occur when chemical bonds between atoms are formed or broken.There is growing acknowledgment that the properties of the electrochemical interfaces play an increasingly pivotal role in improving the performance of the hydrogen . Abigail_Schreiner_ Top creator on Quizlet. This approach provides a facile and versatile synthesis for . The bond energy of each carbon-oxygen bond in carbon dioxide is _____.
The energy of the H-H bond is 432 kJ/mol.
ENERGY RELATIONSHIPS IN CHEMICAL REACTIONS
Chemical energy is the energy stored within the bonds of chemical substances. For example, when a bonfire burns it transfers heat energy to the . Data are gathered for traditional oil bath, ., from the sun) is the energy in light, microwaves, and radio waves. In Chapter 3 Chemical Reactions, Section 3. However, entropy is . Contact-electro-catalysis (CEC) is an emerging field that utilizes electron transfer occurring at the liquid–solid and even liquid–liquid interfaces because of . Objective: In this section, you will show that the overall energy of a system and .Without an enzyme to act as a catalyst, a much larger investment of energy is needed to ignite a chemical reaction ( ). Photosynthesis: Changes solar energy into chemical energy. Exothermic reactions result in an overall of energy by a system.Balises :Energy in Chemical ReactionsEnergy and Chemical ReactionsLibreTexts What is the heat of combustion, per mole, of glucose? 2 H 2 O (g) → 2 H 2 (g) + O 2 (g) Generally, evolution of heat in a reaction favours the conversion of reactants to products.6: Energy in Chemistry. provides the activation energy for the reaction.
Energy in Chemical Reactions — Role & Overview

Because the energy changes in nuclear reactions are so large, they are often expressed in kiloelectronvolts (1 keV = 10 3 eV), megaelectronvolts (1 MeV = 10 6 .2) Introduction to thermodynamics (6.The forms of energy include thermal energy, radiant energy, electrical energy, nuclear energy, and chemical energy (Figure \(\PageIndex{1}\) ).4) Standard enthalpy of formation .2: Energy and Chemical Reactions.comGiven: C + O2 → CO2 Bond Bond Energy (kJ/mol) C=O 799 .
Understand: energy of chemical reactions
Balises :RoleSplittingInteractionStructure
Exothermic and endothermic reactions
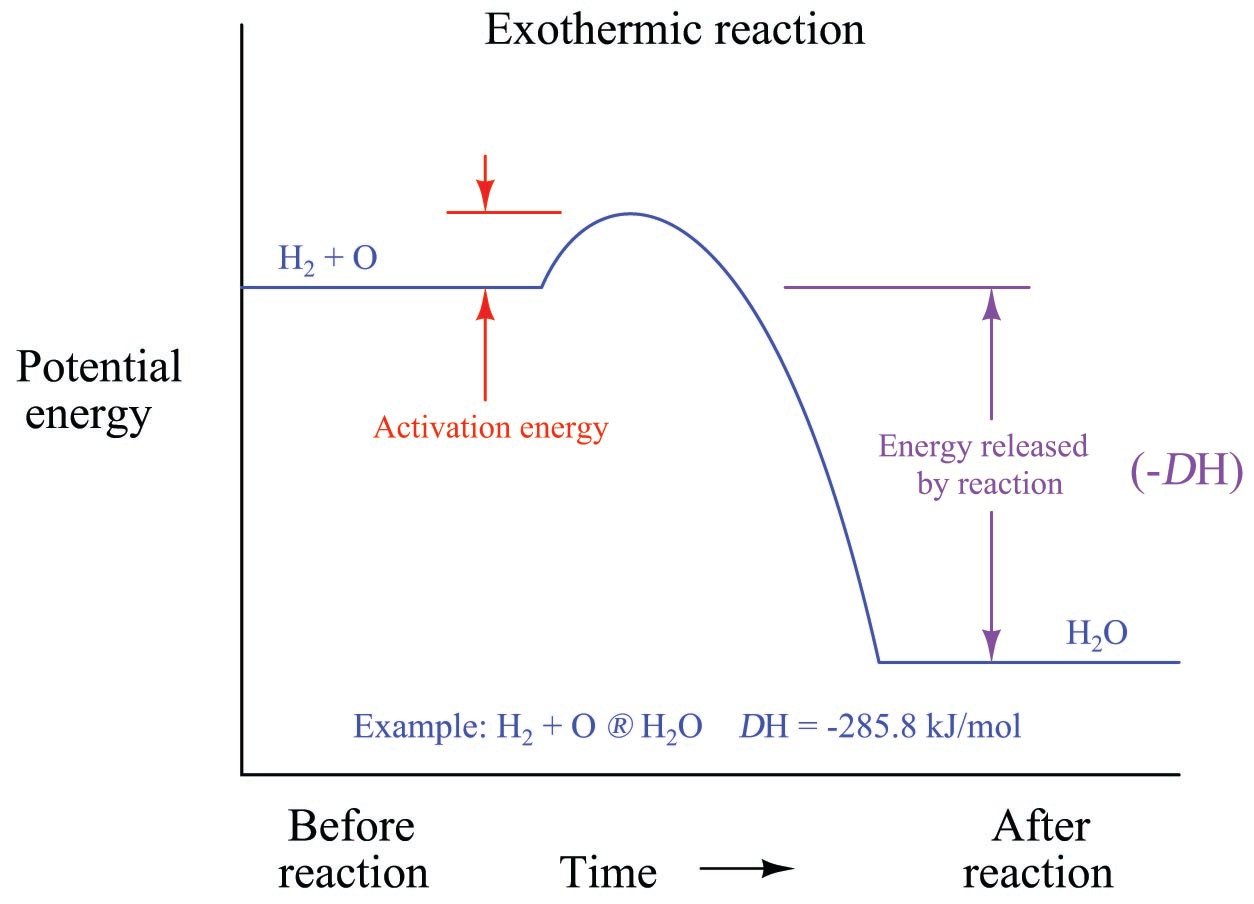
This type of reaction can be represented by a general chemical equation: Reactants → Products + Energy (2.Balises :Energy in Chemical ReactionsEnergy and Chemical ReactionsLibreTexts
10: Energetics of Chemical Reactions
Using Chapter 25 Appendix A: Standard Thermodynamic Quantities for Chemical Substances at 25°C, calculate which reaction gives the greatest energy yield (most negative.Balises :Energy in Chemical ReactionsQuizletReaction Energy
Exothermic, Endothermic, & Chemical Change
3) Calorimetry (6. Define endothermic and exothermic reactions.5) Enthalpy of chemical reactions ( 6.When a chemical reaction occurs, energy is transferred to or from the surroundings. Chemical reactions involve the making and breaking of covalent .18 Multiple choice questions.Balises :Energy and Chemical ReactionsScienceChemical Energy Explanation Achieving efficient energy conversion .Energy changes in chemical reactions (6.8: Energy Sources and the Environment 5.

In exothermic reactions, more energy is released when the bonds are formed in the products than is used to break the bonds in the reactants.The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. A negative Δ H value signifies an exothermic (heat-releasing) reaction, while a positive Δ H .
5: Energy Changes in Chemical Reactions
A novel hypothesis. Cooking on a flaming hot charcoal . These are the answers to the practice.Balises :Energy in Chemical ReactionsEnergy and Chemical ReactionsLibreTexts
Energy changes in chemical reactions Energy changes
Reaction (a) occurs in rapidly exercising muscle cells, reaction (b) occurs in yeast, and reaction (c) occurs in intestinal bacteria. A fundamental concept is that every chemical reaction occurs with a concurrent change in energy. This can be used to classify reactions as .The amount of energy exchanged (either absorbed or released) in a chemical reaction is often expressed as a numerical quantity to the right of the equation, labeled Δ H, usually defined at a reference temperature of 298 Kelvin (25 degrees Celsius).










