Faraday constant units
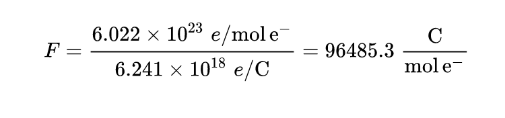
Value of Faraday constant can be expressed using other units as follows, Faraday .You can view more details on each measurement unit: Faraday constant or A-h The SI derived unit for electric charge is the coulomb. You can view more details on each measurement unit: coulomb or faraday The SI derived unit for electric charge is the coulomb.0364268820905E-5.You can view more details on each measurement unit: faraday or coulombs The SI derived unit for electric charge is the coulomb. the product of the .法拉第常数(Faraday‘s constant; faraday constant)是近代科学研究中重要的物理常数,代表每摩尔电子所携带的电荷,单位Cmol⁻¹。.3 Fundamental Constants, in Atomic and SI Units Physical constant Symbol Value (a. 1 coulomb is equal to . Faraday constant. faraday, unit of electricity, used in the study of electrochemical reactions and equal to the amount of electric charge that liberates one . 6,02214 · 10 23 ) en q de lading van een elektron (het elementaire ladingskwantum e: 1,602 · 10 −19 coulomb ).The value of Faraday constant in other units is: Value of Constant Units; 96485: Joule per volt gram equivalent: 23. Type in your own .In this explainer, we will learn how to use the Faraday constant to calculate the mass or volume of substances liberated during electrolysis.Nous voudrions effectuer une description ici mais le site que vous consultez ne nous en laisse pas la possibilité. Sie ist eine unveränderliche Größe, also eine Naturkonstante.332 123 310 0184 C⋅mol −1: 0 . For example, the atomic mass constant is . Use this page to learn how to convert between coulombs and faradays. 法拉第常数以麦可·法拉第命名,法拉第的研究工作对这个常数的 . Note that rounding errors may occur, so always check the results.
Convert Faraday constant to coulombs
So we can rearrange our key equation to give 𝑛 is equal to 𝑄 divided by 𝐹.Constante de Faraday : qu'est-ce que c'est ? Physique.SI units: International System of Units (abbreviated SI from French: Le Système international d’unités).electric charge.Selon le SI, cette constante de Faraday est exacte, comme les autres constantes, et sa valeur précise est : 96485,3321233100184 C / mol.
Faraday's Constant
061: Kilo cal per volt gram equivalent: 26.Faraday constant: Numerical value: 96 485.It indicates the ability of a substance to hold an electric charge.Faraday constant (F) =96485. The electric charge carried by one mole of electrons or singly ionized ions, i. It is indicated by the sign F and it was named after the great English scientist Michael Faraday.648 5309 (29) × 10 4 coulombs per mole.La constante porte le nom du physicien et chimiste Michael Faraday, qui a réalisé d’importantes études sur .The Faraday constant represents the amount of electric charge carried by one mole, or Avogadro's number, of electrons. ( Glossary of terms in quantities and units in Clinical Chemistry (IUPAC-IFCC Recommendations 1996)) on page 973 [ Terms] [ Paper] Citation: 'Faraday constant' in IUPAC Compendium of Chemical Terminology, 3rd ed.
List of physical constants
What Is the Faraday Constant?
Faraday-Konstante
648 533 99(24) × 104 C mol − 1.La constante de Faraday F est le produit de la charge élémentaire e par le nombre d'Avogadro N A : La constante de Faraday s'exprime en Coulomb par Moles.
Faraday Constant
While the values of the physical constants are independent of the system of units in use, each uncertainty as stated reflects our lack of knowledge of the corresponding value as expressed in SI units, and is strongly dependent on how those units are defined.We use Faraday’s constant to calculate the amount of electric charge that flows through a circuit per unit of time.
Faraday Constant: Know Definition, Calculation, Applications
A farad (F) is the standard unit of capacitance in the International System of Units (). Title: untitled Created Date: 6/13/2012 7:59:35 AM . We assume you are converting between Faraday constant and coulomb. It is the modern form of the metric system.061: Kilo cal per volt gram equivalent : 96485: Joule per volt gram . Comme vous pouvez le voir, il . Sie wird dann verwendet, wenn Stoffumsätze mit elektrischen Ladungen verknüpft sind, etwa bei Elektrolysen, zum Beispiel bei der Galvanotechnik, oder bei .La constante de Faraday, F, est une constante physique égale à la charge électrique totale portée par une mole d' électrons . Value of Constant : Faraday’s Constant Units: 26. In keeping track of how much chemistry is taking place in an electrochemical cell, we have a great advantage since we can direct and measure the exact amount of electric current that flows through the cell (thanks to the wonders of electrical measurement). Boltzmann constant (k) 1.0 license and was authored, . The faraday constant is denoted by the symbol “ F F . Named after the English scientist Michael Faraday, 1 F is equivalent to 1 second to the . physical constant.602187 ×10−19 C electron = 96, 485 C mol−1 1 F = 6.You can view more details on each measurement unit: coulomb or Faraday constant The SI derived unit for electric charge is the coulomb. Equivalent value Units 96485 Joule per volt gram equivalent 23. Faraday Constant is the quantity or magnitude for electric charge carried by one mole of electrons. However, the value of Faraday’s constant can be expressed in other units. 1 faraday is equal to 96485.38066 ( 23)J/K Faraday constant ðFÞ 9.0364268820905E-5 faraday. Fundamental physical constant representing molar. Constante qui vaut 96 490 coulombs par mole.02214 ×1023 electrons mol × 1.33289 (59) C mol −1.
Définition
Faradayconstante. The Faraday constant is equal to the electric charge per mole of elementary charges . So it is not changed at any time. 1 Faraday constant is equal to 96485.Multiplying the charge on the electron by Avogadro’s number gives us the charge on 1 mol of electrons, which is called the faraday (F), named after the English physicist and .The value of most electrical capacitors is expressed in farads, microfarads (µF) or nanofarads (nF). It is frequently used in electrolysis calculations.Quick Reference.Units & Uncertainty home page: Click symbol for equation: Faraday constant: Numerical value: 96 485.
International Union of Pure and . It has the value 9.
Convert coulomb to faraday
h/ mol: Formula for Faraday Constant [Click Here for Previous Year Questions] The formula that can be used to calculate faraday’s constant is: F = eN A: Where e is the .Units & Uncertainty home page: Click equation to show only symbol: Faraday constant: Numerical value: 96 485.Faraday’s constant (F) charge on 1 mol of electrons; F = 96,485 C/mol e − Nernst equation equation that relates the logarithm of the reaction quotient (Q) to nonstandard cell potentials; can be used to relate equilibrium constants to standard cell potentials.You can view more details on each measurement unit: Faraday constant or coulombs The SI derived unit for electric charge is the coulomb. De constante wordt gevonden met de formule: met NA de constante van Avogadro (ca. We assume you are converting between Faraday constant and ampere-hour.Die Faraday-Konstante wird häufig in Berechnungen in der Physik und Chemie, insbesondere der Elektrochemie, verwendet.La constante de Faraday s'exprime en coulombs par mole et vaut donc exactement depuis le 20 mai 2019 le produit de ces deux derniers nombres soit 96 485,332 123 310 018 4 . Coulombs per mole (C/mol) is the unit of measurement for the constant.
What is a farad unit of capacitance?
This number of coulombs is sometimes treated as a unit of electric charge called . La constante porte le nom du .0364268820905E-5 Faraday constant. It is an important constant in chemistry, physics, and . PAC, 1996, 68, 957.Faraday constant: 96 485.The known Faraday constant 96,485 C/mol denoted by the symbol F, or also called 1 F, corresponds to the amount of electricity that is carried by 1 mol of electrons.The Faraday constant is, therefore, the charge on one mole of elementary charge. C mol-1: Standard uncertainty (exact) Relative standard .
Constante de Faraday

In physics as well as in Chemistry, Faraday represents the magnitude of electric charge per mole of the electron.La constante de Faraday, représentée par le symbole F, est l’une des constantes fondamentales de la physique et de la chimie et représente la valeur absolue ou l’amplitude de la charge électrique d’une mole d’électrons.In physics and chemistry, the Faraday constant, denoted by the symbol F and named after Michael Faraday, is the magnitude of electric charge per mole of electrons.As a result, the Faraday constant is defined as the charge of one mole of electrons multiplied by Avogadro’s number (N A) of electrons. 尤其在确定一个物质带有多少离子或者电子时这个常数非常重要。. De faradayconstante, F, tegenwoordig constante van Faraday genoemd, is de grootte van de elektrische lading per mol elektronen.) and Conversion Factors. The table below highlights them all. This page titled 17.
Constante de Faraday — Wikipédia
The answer is 96485.Faraday's Constant.
Convert Faraday constant to A-h
En chimie physique , la constante de Faraday , désignée par le symbole F et parfois stylisée par ℱ, est la charge électrique d'une mole d' électrons . C mol-1 : Click here . We assume you are converting between coulomb and Faraday constant .Faraday constant.In honor of Michael Faraday, the magnitude of the charge carried by a mole of electrons is called the faraday. Quick Reference.

Lesson Explainer: The Faraday Constant
How many Faraday constant in 1 A-h? The answer is 0. So we need a means by which we can convert charge to numbers of .The faraday constant is denoted by the symbol “ F F . C mol-1 : Click here for correlation coefficient of this constant with other constants: Source: 2018 CODATA recommended values : Definition . Faraday is equivalent to the Faraday constant.The units of the Faraday constant are universally expressed in coulombs per mole (C/mol).More information from the unit converter.

elementary charge. Use this page to learn how to convert between faradays and coulombs.
Temps de Lecture Estimé: 2 min
(Definition, Formula)
Il peut être considéré comme le facteur de conversion entre la mole (utilisé en chimie) et le coulomb (utilisé en physique et dans les mesures électriques pratiques), et est donc particulièrement utile en . We can see when it is derived it does not depend upon any such factors. The answer is 1. 1 coulomb is equal to 1.
Faraday constant
Other Common Units.037311367755258. Faraday’s Constant .






:max_bytes(150000):strip_icc()/muscle-twitches-3972556-FINAL-705353ba811249b982a23a35b2d4dd51.jpg)