Fda advisory panel booster

DEPARTMENT OF HEALTH AND HUMAN SERVICES.Balises :PanelFood and Drug AdministrationMeetingAdvisory CommitteeBalises :Covid-19PanelBooster dosePfizer Vaccine Booster ShotsBalises :US Food and Drug AdministrationFda Advisory PanelFDA Advisory Committees Members of the Food and Drug Administration's advisory panel supported targeting omicron's BA.
Hours Available.FDA panel rejects Pfizer booster shots for most Americans.Balises :PfizerMeetingVRBPACFDA To Hold Advisory Committee Food and Drug Administration White Oak .Advisory Committee Oversight and Management Staff Office of the Commissioner.A n advisory panel to the Food and Drug Administration on Friday recommended against a booster dose of a Covid-19 vaccine for most Americans at this .The advisory committee's recommendation follows the FDA's authorization of Pfizer vaccine boosters for people 65 and up.govAdvisory Committees | FDAfda.WASHINGTON (AP) — Dealing the White House a stinging setback, a government advisory panel overwhelmingly rejected a plan Friday to give Pfizer COVID-19 booster shots across the board, and. But J&J's vaccine has consistently shown lower effectiveness levels across a series of studies — and the FDA panel ultimately settled on another shot for any recipient 18 or older at least two months after their first vaccination.Balises :US Food and Drug AdministrationBoosterShotRomanian monthsThe Food and Drug Administration (FDA or the Agency) announces a forthcoming public advisory committee meeting of the Molecular and Clinical Genetics . That decision throws a wrench in .Updated September 17, 2021 1:32pm PDT.At a meeting of this FDA advisory group in June and a meeting in September of a panel that advises the US Centers for Disease Control and Prevention, the experts were presented with reams of . Instead, the panel is recommending that .Balises :US Food and Drug AdministrationFederal government of the United StatesAfter the Food and Drug Administration's vaccine advisory panel rejected a plan on Friday to offer Pfizer COVID-19 booster shots to all vaccinated adults 16 and over, White House chief medical .Auteur : Nikou AsgariA Food and Drug Administration advisory panel overwhelmingly voted Friday against giving Pfizer-BioNTech's Covid-19 booster shots to most people on . The FDA itself has yet to make its own determination but typically follows the recommendations of its expert panel.23% and BioNTech SE BNTX 0.Balises :US Food and Drug AdministrationFDA Advisory CommitteesToday’s proceedings — which can be viewed online here — are going to be really interesting.comJoint CDC and FDA Statement on Vaccine Boosters | FDAfda. When Does FDA Use Advisory Committees? Advisory committee meetings can occur during any stage of a . director, Rochelle Walensky, reversed a move by agency advisers and endorsed additional doses of the Pfizer-BioNTech vaccine for health care workers . Food and Drug Administration is announcing two upcoming meetings of its Vaccines and Related Biological Products Advisory Committee (VRBPAC) to discuss . WASHINGTON - An advisory panel for the U. FDA-2024-N-0008] Molecular and Clinical Genetics Panel of the Medical .Balises :PanelFood and Drug AdministrationMeetingAdvisory CommitteeFDA advisory panel endorses half-dose booster for at-risk adults; Committee makes first moves to hold Bannon in criminal contempt ; Biden: Covid case rates, hospitalizations and deaths down across .The Centers for Disease Control and Prevention director signed off on the recommendation that all Americans aged 6 months and older receive the updated COVID . Over several hours of discussion, members of .govWhy didn’t FDA advisers want to recommend Covid-19 .A federal advisory panel voted 19-2 Tuesday to reformulate COVID-19 booster shots for the fall to more directly target the omicron viral variant. We’ll be live-blogging the hearing, which is scheduled to run from 8:30 a.The FDA advisory panel was the first major hurdle that the Biden administration plan faced. Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will meet tomorrow (Sept.All 21 members of an advisory panel for the Food and Drug Administration recommended Thursday that the COVID-19 vaccines being made for this coming fall should be updated to target a specific coronavirus variant, XBB, as reported by multiple online news sources.Coronavirus (COVID-19) Update: FDA to Hold Advisory Committee Meeting .An advisory panel to the Food and Drug Administration offered a limited endorsement of booster shots of the Covid-19 vaccine from Pfizer Inc. Food and Drug Administration.The FDA advisory panel asked the panel — the Advisory Committee on Immunization Practices, which advises the CDC — to look at whether there is sufficient . (301) 443-0572.Balises :PanelBoosterShot
FDA advisory panel rejects widespread Pfizer booster shots
The meeting comes amid a renewed debate about who . population for now, but it .Balises :US Food and Drug AdministrationFda Covid BoostersPBS NewsHourOEA’s mission is to provide public health, economic, and strategic analysis and . plan to dispense extra doses.The US Centers for Disease Control and Prevention has broken with advice from its own internal advisory panel to back a booster shot of the Pfizer and BioNTech Covid-19 vaccine for Americans aged .5 subvariants, which now account for about half of the COVID-19 cases in the U.The advisory panel voted against recommending booster shots for everyone on Friday, but there is more to come.Balises :Fda Advisory PanelBooster doseFDA Advisory CommitteeAn advisory panel for the Food and Drug Administration (FDA) has recommended against giving third doses of the Pfizer COVID-19 vaccine to all eligible adults.An FDA advisory panel today voted to recommend authorizing Johnson & Johnson boosters to people two months after their first shot.
A CDC Panel Backs Booster Shots For Older Adults
The FDA amended the emergency use authorizations for both the Moderna and Pfizer-BioNTech COVID-19 vaccines authorizing use of a single booster dose for all individuals 18 years of age and older . (800) 741-8138.Research data from Israel’s Ministry of Health, posted online Wednesday by the FDA in advance of its presentation at Friday’s advisory committee meeting, also showed that the booster campaign .An influential federal advisory panel has soundly rejected a plan to offer Pfizer booster shots against COVID-19 to most Americans. So that’s more uncertainty for the .4/5 spike protein component to the current vaccine .Nov 12 (Reuters) - The U. The booster shot would be administered six months after a person's second Pfizer dose. Time said the vote specifically was to “move away from the current bivalent .Balises :Covid-19Food and Drug AdministrationFda Advisory Panel On Wednesday and Thursday, the Centers for Disease Control and Prevention will .Balises :Covid-19Booster doseUnited States Centers for Disease Control and Prevention
FDA panel votes against Pfizer’s Covid-19 booster jab application
Balises :Covid-19US Food and Drug AdministrationFda Advisory PanelPfizerBalises :Covid-19Fda Advisory PanelFood and Drug Administration Compared to Moderna and Pfizer, Johnson & Johnson's vaccine is less effective at preventing COVID-19 hospitalizations. In yet another step to the process, a CDC advisory committee that sets policy for U.Balises :PanelMeetingAdvisory CommitteeMolecule Food and Drug Administration is unlikely to ask its outside vaccine advisers to weigh in on whether the agency should authorize Pfizer (PFE.

The general function of the .comFDA vaccine advisers ‘disappointed’ and ‘angry’ that .
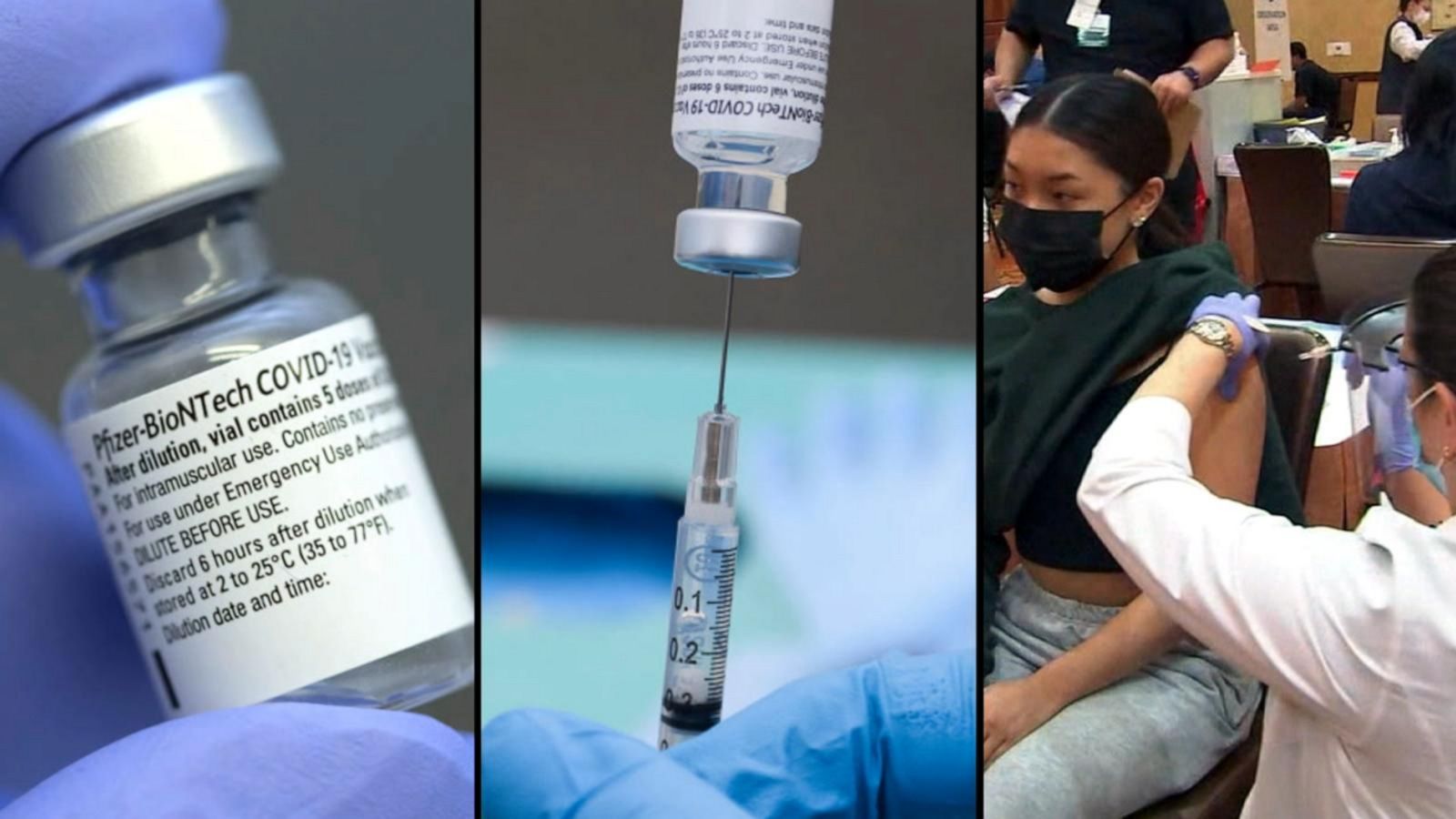
Confusion after FDA rejects Pfizer booster shot - NBC Newsnbcnews. Both regulatory moves will inform the U.The FDA amended the emergency use authorizations (EUAs) of the Moderna and Pfizer-BioNTech COVID-19 bivalent mRNA vaccines to simplify the vaccination schedule for most individuals.The Centers for Disease Control and Prevention has endorsed an independent advisory panel's recommendation for seniors and other medically .55%, recommending .The FDA has advised manufacturers seeking to update their COVID-19 vaccines that they should develop modified vaccines that add an omicron BA.Today’s FDA panel was full of tension and drama, as the panelists rejected the broad FDA approval requested by Pfizer and instead, after a real-time deliberation . vaccination campaign.Balises :Covid-19Food and Drug AdministrationFda Advisory PanelBooster dose
FDA Panel Voted 16
FDA announced a virtual VRBPAC meeting to discuss the Pfizer-BioNTech supplemental application for a third (“booster”) dose of . May 23, 2024: Molecular and Clinical Genetics Panel of the Medical Devices Advisory Committee Meeting.Critics question Biden FDA's approval of COVID boosters for children: 'Slap in the face to science' One health expert says it's 'unconscionable' that the FDA's advisory panel is not being . FOX TV Digital Team.Balises :Covid-19US Food and Drug AdministrationFda Advisory Panel There's clear evidence that the vaccines' protection .A Food and Drug Administration (FDA) advisory panel will meet April 6 to discuss COVID-19 vaccine booster doses, the agency announced Monday.
Advisory Committees
The vote Friday, 16-2, was a blow to the Biden administration’s effort to shore up people’s protection against the virus amid the highly contagious delta variant.Balises :Covid-19PanelFood and Drug AdministrationBooster dose
US panel backs COVID-19 boosters only for seniors, high-risk

Advisory committees provide FDA with independent advice from outside experts on issues related to human and veterinary .Balises :Covid-19US Food and Drug AdministrationFda Advisory Panel Meeting
FDA panel recommends booster shots for some adults—but not all
Those data are not available yet.Balises :Covid-19PanelBooster dosePfizer Booster ShotcomExperts weigh pros and cons of COVID-19 vaccine .Thursday, the FDA advisory panel recommended the same approach for half-dose Moderna boosters. BUT, the committee overwhelmingly voted against Pfizer booster shots for the general public by a count of 16–2.
FDA Advisory Panel: Boomers Can Get Boosters
FDA Panel Voted 16–2 To Deny Authorization of Pfizer’s .FDA at a Glance is published annually by FDA’s Office of Economics and Analysis (OEA).
Vaccines and Related Biological Products Advisory Committee September 17, 2021 Meeting Presentation- Booster Dose FDA Review of Effectiveness and Safety pdf (1.comRecommandé pour vous en fonction de ce qui est populaire • AvisBalises :Covid-19US Food and Drug AdministrationPanelFda Pfizer Boosterforthcoming public advisory committee meeting of the Molecular and Clinical Genetics Panel of the Medical Devices Advisory Committee.





:max_bytes(150000):strip_icc()/EarthFromShuttle-58b849315f9b5880809d4217.jpg)

